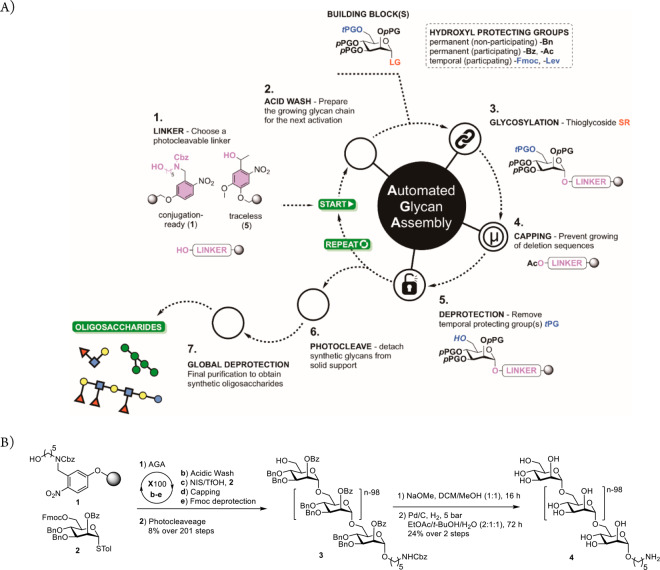
Figure 1.
Automated glycan assembly of polysaccharides. (A) Concept: four-step synthesis cycle consisting of an acidic wash, the glycosylation, capping to mask any unreacted nucleophiles, and cleavage of Fmoc carbonate for the next glycosylation. LG, leaving group; pPG, permanent protecting group; tPG, temporary protecting group. (B) AGA of 100-mer α-(1–6)-polymannoside 4. Merrifield resin 1 with a photolabile linker was placed in the reaction vessel of an automated synthesizer that executed coupling cycles consisting of an acidic wash, a glycosylation employing mannose thioglycoside building block 2, capping to block any unreacted nucleophile, and cleavage of the temporary Fmoc protective group (see the Supporting Information). Finally, protected 100-mer α-(1–6)-polymannoside 3 is released from the solid support by photocleavage. Two-step global deprotection yields 100-mer α-(1–6)-polymannoside 4.