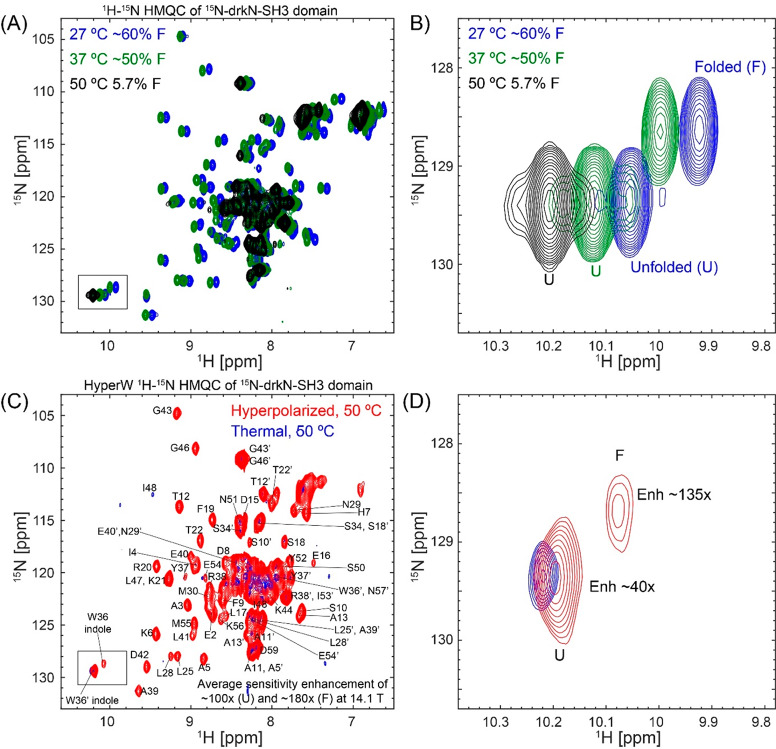
Figure 7.
SH3 folded and unfolded states visualized by HyperW HMQC. (A) 2D 1H–15N HMQC spectra measured for a ∼520 μM 15N-drkN-SH3 domain in an 87.4% H2O buffer (50 mM HEPES buffer, 50 mM KCl, pH 7.5) at 27 °C (blue), 37 °C (green), and 50 °C (black). Indirect- and direct-domain spectral widths of 9014.4 and 2312.7 Hz were covered, using 64 hypercomplex t1 increments.34 The flip angle of the selective excitation was 90°, and 16 scans were collected using a 56.8 ms acquisition time and a relaxation delay of 2 s. Total experimental time was 1 h 12 min. (B) Enlarged region of Trp36 indole peak (marked with a black rectangle in (A)) showing the thermally driven rise of the unfolded state. (C) Comparison between 2D HyperW (red) and thermally polarized (blue) 1 H–15N HMQC spectra measured for the 15N-drkN-SH3 domain. 2.8 mL of buffered D2O (50 mM HEPES, pD 7.5, 50 mM KCl) was used to dissolve an 85/15 water/glycerol pellet containing 10 mM 4-amino-TEMPO. The pellet was polarized at 1.17 K for 3 h 30 min using 100 mW microwave irradiation at 94.195 GHz. ∼180 μL of the resulting hyperpolarized water solution was injected into a 5 mm NMR tube containing ∼140 μL of a ∼1.3 mM 15N-drkN-SH3 solution. Partial assignment of the various residues indicated by single-letter amino acid codes is done on the basis of Zhang et al.65 Resonances of the folded conformation are labeled with these assignments, and resonances of the unfolded form are marked with an added prime (′). These spectra were recorded at 50 °C using 64 hypercomplex t1 increments covering indirect- and direct-domain spectral widths of 7211.5 and 1947.5 Hz. The HyperW spectrum was recorded using two phase-cycled scans per t1 increment. Total experimental time was 63 s for the hyperpolarized spectrum (acquisition time of 177.5 ms, repetition delay of 0.037 s) and 13 h 50 min for the thermal spectrum (176 scans, acquisition time of 177.5 ms and a repetition delay of 2 s per t1 increment). (D) Enlarged region of the Trp36 indole peak (marked with a black rectangle in (C)).