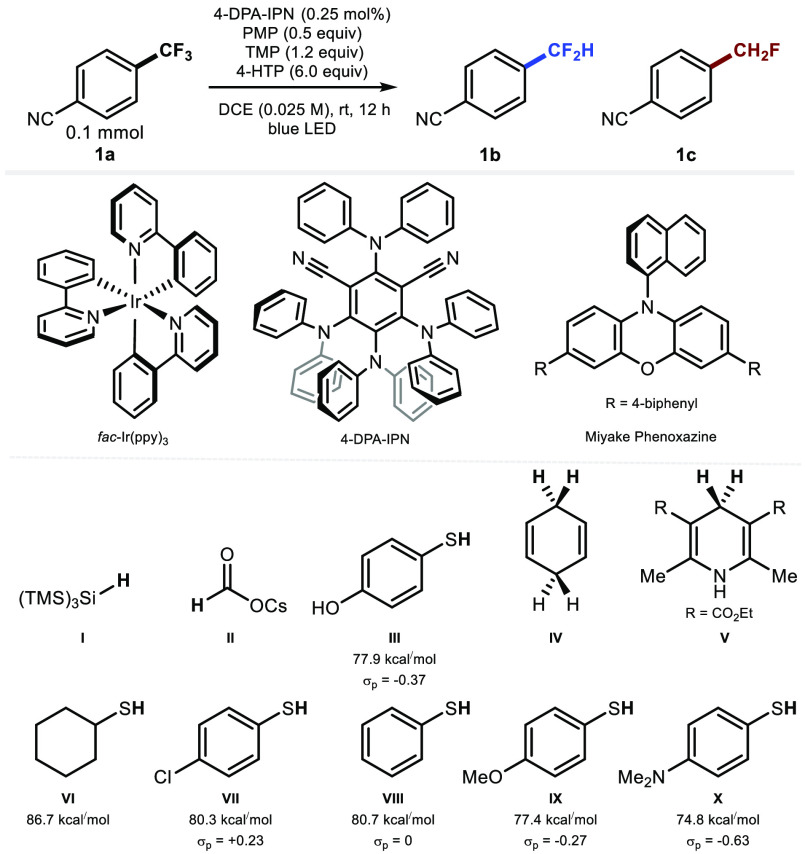
Table 1. Experiments for the Hydrodefluorination of 4-(Trifluoromethyl)benzonitrile 1a.
| Entry | Alterations to conditions | Yielda(ratio 1b:1c) |
|---|---|---|
| 1 | Mg0 (30 equiv), H2O/AcOH/DMSO | 0% |
| 2 | Pd(OAc)2 (3 mol %), CuF2 (20 mol %), 2-pyridone (5 mol %), KOSiMe3 (7.0 equiv), DMF, 45 °C, then tBuOH (2.0 equiv), 60 °C | 0% |
| 3b | Miyake Phenoxazine (2 mol %), II (3 equiv), blue LED, DMSO, 50 °C, 24 h | 4% (2:1) |
| 4c | fac-Ir(ppy)3 (1.0 mol %), TMP (2.0 equiv), HBPin (3.0 equiv), blue LED, DCE, rt, 24 h | 0% |
| 5d | PTH (10 mol %), VI (10 mol %), II (3 equiv), blue LED, 5% H2O/DMSO, rt, 24 h | 0% |
| 6 | fac-Ir(ppy)3 (2.5 mol %) | 53% (5:1) |
| 7 | 4-DPA-IPN (2.5 mol %) | 62% (5:1) |
| 8 | No alteration | 65% (5:1) |
| 9 | I (6 equiv) instead of III | trace |
| 10 | II (6 equiv) instead of III | trace |
| 11 | IV (6 equiv) instead of III | 0% |
| 12 | V (6 equiv) instead of III | 15% (5:1) |
| 13 | VI (6 equiv) instead of III | 4% (8:1) |
| 14 | VII (6 equiv) instead of III | 4% (8:1) |
| 15 | VIII (6 equiv) instead of III | 5% (8:1) |
| 16 | IX (6 equiv) instead of III | 22% (7:1) |
| 17 | X (6 equiv) instead of III | 22% (7:1) |
| 18 | no PMP | 51% (5:1) |
| 19 | no TMP | 31% (>20:1) |
| 20 | no TMP and no PMP | 0% |
| 21 | no light | 0% |
| 22 | no 4-HTP | 0% |
| 23 | no photocatalyst | 0% |
Combined yields of 1b and 1c determined by 19F NMR using 4-fluoroanisole as internal standard; the ratio of 1b:1c is given in parentheses.
Reaction carried out on 14a.
Conditions of ref (7c) with no alkene.