Scheme 5. Biologically Active Sulfoxide Reduction,
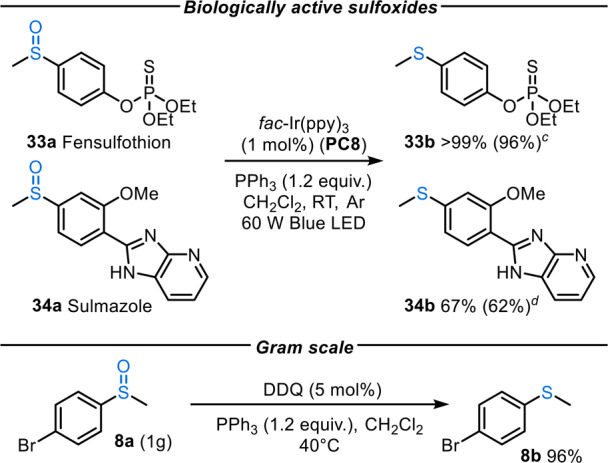
Reaction conditions: sulfoxide (0.30 mmol), fac-Ir(ppy)3 (1 mol %), PPh3 (0.36 mmol) in CH2Cl2 (1.5 mL) at RT.
1H NMR yields reported based on a trimethoxybenzene internal standard and isolated yields of products after column chromatography are shown in parentheses.
48 h reaction time.
24 h reaction time.
