Abstract
Clostridium difficile infection (CDI) is a prevalent nosocomial and increasingly community-acquired problem. Little is known about the productive cellular response in patients. We used flow cytometry to define inflammatory (Th1 and Th17) and regulatory [Foxp3+ T-regulatory (Treg)] cells present in circulating peripheral blood mononuclear cells (PBMC) from CDI patients. We consented 67 inpatients that tested either positive or negative for CDI and 16 healthy controls and compared their PBMC phenotypes. PBMC were collected, isolated, and stained for CD3, CD8 and either IL17 (Th17), IFN-γ (Th1) or Foxp3 (Treg) and analysed using flow cytometry. Twenty thousand events were collected in the lymphocyte gate (gate 1) and T-cell phenotypes were defined. CDI patients who clear the primary initial infection have greater numbers of non-CD3 PBMC. CDI patients who develop recurrence of CDI have a greater percentage of CD3+CD8+, CD3+CD4+Foxp3 and fewer low granular CD3−Foxp3+ PBMC. These patients have greater numbers of IFN-γ-producing lymphocytes, as well as PBMC phenotypes represented by increased IFN-γ- and IL17-co-expressing CD4+CD3+. This initial pro-inflammatory phenotype decreases with repeated recurrence, demonstrating importance of timing of sample collection and history of symptoms. Patients with a history of recurrence had increased Foxp3+CD3+CD4+ and IL17+CD3+CD4+ populations. Hence, CDI recurrence is hallmarked by greater numbers of circulating CD3+ lymphocytes skewed towards a Th1/Th17 inflammatory population as well as possible immune plasticity (Th17/Treg).
Introduction
In 2008, the Infectious Diseases Society of America (IDSA) issued notification of a forthcoming epidemic of antibiotic resistant infections (Spellberg et al., 2008). In their 2013 report, the United States Centers for Disease Control and Prevention (CDC) singled out Clostridium difficile infection (CDI) as one of three urgent health threats due to overuse and antibiotic resistance (CDC, 2013). At the time of writing, it is estimated that 250 000 people will contract CDI and 14 000 will die this year from the infection. The cost of this infection to the healthcare system will be over one billion dollars annually (Spellberg et al., 2008). To curb the growth and cost of CDI, we need to better understand how the host responds and what constitutes the most productive form of response, including both humoral and cellular arms of immunity.
The clostridial species are involved in education of the immune system as well as contributing to the homeostasis of the intestinal tract (Atarashi et al., 2011; Chiba & Seno, 2011; Gaboriau-Routhiau et al., 2009). Naturally occurring toxigenic C. difficile can be found in 1–3 % of adults while both non-toxigenic and toxigenic forms transiently colonize 70–100 % of infants during their first year of life (Adlerberth et al., 2014; Rousseau et al., 2012). The optimal response for clearing CDI requires both humoral and cellular immunity (Kelly & Kyne, 2011; Madan & Petri, 2012). It is known that recurrent CDI patients produce a diminished humoral response to both toxin and non-toxigenic antigens (Kelly, 2012; Madan & Petri, 2012; Péchiné et al., 2007; Yacyshyn &Yacyshyn, 2013). In a recent publication, increased levels of faecal IL8 and CXCL-5, markers of intestinal inflammation, demonstrated greater correlation to remitting or persistent CDI than to bacterial burden (El Feghaly et al., 2013a, b). Hence previous studies suggest that recurrent CDI patients’ immune response is less than optimal for bacterial clearance and potentially skewed towards producing intestinal inflammation. We hypothesized that recurrent patients produce a greater proinflammatory response during CDI, which could be detected in the Th17, Th1 and T-regulatory (Treg) subsets circulating in their peripheral blood mononuclear cells (PBMC).
Methods
Patients with C. difficile: inclusion/exclusion criteria.
All patients positive for C. difficile were reviewed at the University of Cincinnati hospital between Jan 2011 and Sept 2012. Inclusion criteria: adults at least 18 years of age, have had at least one ELISA+toxin test or a positive loop-mediated isothermal amplification (LAMP) test within 72 h, and agreed informed consent. All had started standard of care (SOC) therapy, either metronidazole or vancomycin. Exclusion criteria: patients having cancer, on chemotherapy, taking any immunosuppressive, major surgery within 6 weeks, HIV+ or any chronic GI inflammatory disease.
Definitions.
Initial CDI was defined as new onset of diarrhoea (≥3 loose stools day−1 for more than 24 h), at least one positive laboratory test and no other C. difficile positive test within 1 year of surveillance. This patient group hereafter is referred to as ‘initial’. Recurrent CDI was defined as recurrence or onset of new diarrhoea after a symptom-free period of ≥3 days and completion of at least one round of SOC therapy. We consented 32 patients at the point of new onset CDI. Six of these patients became recurrent and hereafter are referred to as ‘became recurrent’. Due to excluding over 50 % of CDI patients for factors previously mentioned and a set timeline for patient enrolment, we included these six CDI patients in the recurrent group. Upon initial analysis, it was apparent that those patients who ‘became recurrent’ (6 CDI patients) were different from the ‘known recurrent’ patients (14 CDI patients). Case controls were comorbid and hospitalized patients who had diarrhoea and were tested for CDI but were negative and remained negative over the 1 year follow up. Over the course of patient enrolment, two types of toxin tests were used. From November 2010 to August 2011, the enzyme immunoassay for toxins A and B was used. From August 2011 on, the Meridian Illumigene for C. difficile was used. Healthy controls were healthy individuals who were asked and consented to give blood, medical history and concomitant medications.
Patient medical history and concomitant medications.
The history and medication lists of each patient were recorded on the day of consent and blood was drawn. We amalgamated over 100 different medical diagnoses and allocated them amongst 13 systemic medical systems. Case controls and initial patients exhibited a mean of nine different medical diagnoses, while the recurrent population exhibited a mean of 10. Almost 150 different concomitant medications were incorporated into a list resulting in 23 top medication classes. All three patient groups were receiving a mean of 14 different medications (including vitamins).
PBMC isolation and flow cytometry.
PBMC were isolated from heparinized blood on Ficoll-Hypaque and divided into two portions. PBMC (1×106 ml−1) were stimulated with PMA (50 ng ml−1)/ionomycin (1 μg ml−1) and RPMI 1640, 10 % heat inactivated FBS, supplemented with penicillin and streptomycin. RPMI diluent was added to control cultures to maintain similar volumes and incubated side by side with the PMA/ionomycin-stimulated cultures. Golgistop (2 μl per 3 ml of media) was added to each culture (both PMA and control cultures). After 4 h, cells were collected, stained for CD8/CD3 and either Foxp3 (Treg), IL-17 (Th17) or IFN-γ (Th1) and analysed on an Accuri C6 flow cytometer for surface and intracellular expression of the markers. For analysis, 20 000 events were captured in the lymphocyte gate (gate 1). We also analysed cells found in gate 2, a cell population that contained cells outside the lymphocyte gate, with a larger volume (forward scatter, FSC) and granularity (side scatter, SSC), more typically associated with macrophages. Flow data were analysed using FCS Express (De novo Software). CD4 cells were identified as CD3+CD8− cells, as stimulation with PMA/ionomycin decreases/abrogates CD4 expression after activation, making direct staining of CD4 unreliable (Mao et al., 2004; Pelchen-Matthews et al., 1993). Antibodies purchased from BD Bioscience and used for flow cytometry were: anti-CD3 PerCP-Cy5.5, anti-CD8 APC, anti-Foxp3 PE, anti-IFN-γ PE and anti-IL17a PE. PerCP Cy5.5-, APC- and PE-labelled control Ig antibodies were also purchased from BD Bioscience.
Statistical analysis.
Data were analysed using non-parametric methods. Flow cytometry data were first analysed using the Kruskal–Wallis one-way ANOVA, followed by Wilcoxon rank sum analysis or Spearman rank correlations. Medical history and concomitant medicine demographic information were compared using two by two tables and the Fisher exact test. Statistix 9 statistical software was used for analysis.
Results
Patient demographics
Thirty-two patients who presented with initial CDI were consented and entered the study. Six patients were later excluded for other reasons, leaving 26 initial CDI patients. However, 6 of the 26 initial CDI patients became recurrent within 1–3 months, giving us an approximate 19 % recurrence rate during our study. A further 14 recurrent patients were consented upon presentation of new diarrhoea after a symptom-free period of ≥3 days and completion of at least one round of SOC therapy. Twenty-one comorbid case controls (CDI negative) were consented and 20 entered the study. Sixteen healthy controls were also consented and included in the study. General age, gender and race distribution can be seen in Table 1. Patients’ comorbidities are found in Table 2. More of the recurrent population presented with anaemia and rheumatoid comorbidities. The case controls appeared to have more urinary tract or kidney problems. In Table 3, we illustrate the wide variations in patient concomitant medications. The mean number of current medications per patient group was 14, ranging from 6 to 30 medications for individuals. Several patients had complex medication profiles, with multiple medications in a single class. However, for representation, these were aggregated and only counted as one. Interestingly, more case controls were on laxatives.
Table 1.
Patient demographics
Patient demographics were collected and recorded on day of consent.
| Demographic | Healthy control (n = 16) | Case control (n = 20) | Initial CDI (n = 20) | Recurrent CDI (n = 20) |
| Age (mean) | 48.43 | 52.8 | 59.80 | 52.90 |
| Age (range) | 30–62 | 25–77 | 30–79 | 33–84 |
| Proportion of group with age ≥65 (%) | 0 | 25 | 45 | 25 |
| Female gender | 81 % (14/16) | 70 % (14/20) | 55 % (11/20) | 60 % (12/20) |
| Caucasian | 16 | 11 | 13 | 11 |
| African American | 0 | 9 | 7 | 9 |
Table 2.
Patient comorbidities
Patient histories were recorded on the day of consent. Patients could have complex histories with more than one comorbidity associated with a system. However, for ease of representation and comparison the comorbidities were aggregated and counted as one in each system.
| System | Healthy control (%) (n = 16) | Case control (%) (n = 20) | Initial CDI (%) (n = 20) | Recurrent CDI (%) (n = 20) |
| Cardiovascular | 6.2 (1/16) | 75 (15/20) | 70 (14/20) | 70 (14/20) |
| Endocrine | 6.2 (1/16) | 70 (14/20) | 60 (12/20) | 55 (11/20) |
| Gastrointestinal | 18.8 (3/16) | 65 (13/20) | 70 (14/20) | 80 (16/20) |
| Haematology/oncology | 0 (0/16) | 5 (1/20) | 15 (3/20) | 10 (2/20) |
| Anaemia | 0 (0/16) | 20 (4/20)* | 25 (5/20) | 55 (11/20) |
| Infection (non-C. difficle) | 0 (0/16) | 30 (6/20) | 25 (5/20) | 35 (7/20) |
| Musculoskeletal | 25 (4/16) | 20 (4/20) | 25 (5/20) | 30 (6/20) |
| Neurological | 6.2 (1/16) | 35 (7/20) | 25 (5/20) | 35 (7/20) |
| Psychiatric | 6.2 (1/16) | 35 (14/20) | 35 (7/20) | 45 (9/20) |
| Rheumatoid | 12.5 (2/16) | 5 (1/20)† | 10 (2/20) | 35 (7/20) |
| Respiratory | 18.8 (3/16) | 35 (7/20) | 40 (8/20) | 65 (13/20) |
| Urinary tract or kidney | 0 (0/16) | 60 (12/20)ठ| 25 (5/20) | 20 (4/20) |
| Age-adjusted Charlson comorbidity index | 3.05 | 3.9 | 3.9 |
Case control vs recurrent CDI P = 0.048.
Case control vs recurrent CDI P = 0.0436.
Case control vs recurrent CDI P = 0.022.
Case control vs initial CDI P = 0.053.
Table 3.
Patient concomitant medications
A patient’s concomitant medications were recorded on day of consent. Patients could be on multiple medications in one category; however, for ease of representation and comparison of groups, these were aggregated and counted as one for each category.
| Drug category | Healthy control (%) (n = 16) | Case control (%) (n = 20) | Initial CDI (%) (n = 20) | Recurrent CDI (%) (n = 20) |
| ACE inhibitor | 0 (0/16) | 25 (5/20) | 30 (6/20) | 20 (4/20) |
| Beta-blocker | 6.2 (1/16) | 60 (12/20) | 35 (7/20) | 35 (7/20) |
| Probiotic | 0 (0/16) | 20 (4/20) | 25 (5/20) | 40 (8/20) |
| Proton pump inhibitor | 18.8 (3/16) | 55 (11/20) | 70 (14/20) | 55 (11/20) |
| Statin | 0 (0/16) | 50 (10/20) | 20 (4/20) | 30 (6/20) |
| Allergy medications | 6.2 (1/16) | 30 (6/20) | 25 (5/20) | 20 (4/20) |
| Anti-epileptics | 6.2 (1/16) | 40 (8/20) | 35 (7/20) | 30 (6/20) |
| Blood thinner | 6.2 (1/16) | 65 (13/20) | 65 (13/20) | 60 (12/20) |
| Diuretic | 6.2 (1/16) | 15 (3/20) | 35 (7/20) | 25 (5/20) |
| Narcotics | 6.2 (1/16) | 45 (9/20) | 40 (8/20) | 60 (12/20) |
| Pain (non-narcotic) | 12.5 (2/16) | 30 (6/20) | 30 (6/20) | 20 (4/20) |
| Stool laxative | 6.2 % (1/16) | 45 % (9/20) | 25 % (5/20) | 10 % (2/20)* |
| SSRI/SNRI† | 25 % (4/16) | 35 % (7/20) | 30 % (6/20) | 25 % (5/20) |
| Thyroid medications | 6.2 % (1/16) | 35 % (7/20) | 10 % (2/20) | 20 % (4/20) |
| Antibiotics/antifungals | 0 % (0/16) | 40 % (8/20) | 100 % (20/20)‡ | 100 % (20/20)‡ |
| Anti-emetic | 0 % (0/16) | 45 % (9/20) | 25 % (5/20) | 25 % (5/20) |
| Anti-psychotic | 0 % (0/16) | 20 % (4/20) | 15 % (3/20) | 10 % (2/20) |
| Benzodiazepine | 0 % (0/16) | 45 % (9/20) | 30 % (6/20) | 35 % (7/20) |
| Insulin | 0 % (0/16) | 50 % (10/20) | 35 % (7/20) | 45 % (9/20) |
| Gastrointestinal prokinetic | 0 % (0/16) | 20 % (4/20) | 25 % (5/20) | 15 % (3/20) |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | 25 % (4/16) | 0 % (0/20) | 5 % (1/20) | 5 % (1/20) |
| Respiratory medication | 0 % (0/16) | 45 % (9/20) | 40 % (8/20) | 40 % (8/20) |
| Vitamins/minerals | 43.7 % (7/16) | 60 % (12/20) | 70 % (14/20) | 75 % (15/20) |
P<0.05 Recurrent vs case controls.
Selective serotonin reuptake inhibitors/serotonin–norepinephrin reuptake inhibitors.
P<0.0001 Recurrent vs case control and initial vs case control.
Quantitative PBMC differences
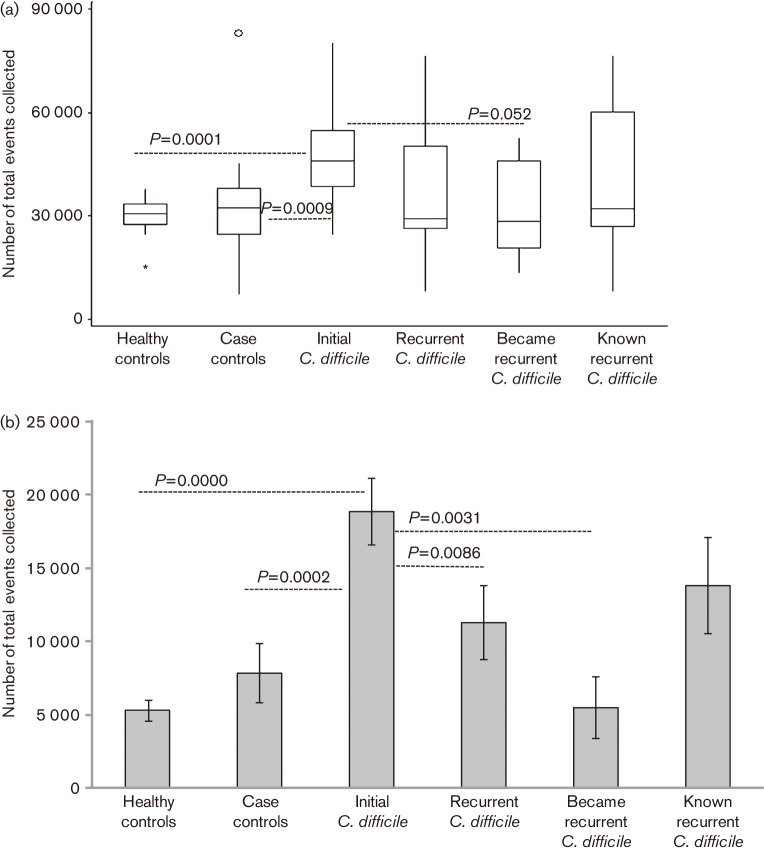
Hospitalized CDI patients can present with increased white blood count due to acute infection (Bulusuet al., 2000; Planche, 2013). Since our original goal was to determine if qualitative differences in circulating T-cell populations were present in PBMC, we collected 20 000 events in the standard lymphocyte gate (gate 1) for each individual sample. Surprisingly, we detected a quantitative difference between the four groups. We consistently needed to collect more cells to reach 20 000 events in gate 1 from initial patients (Kruskal–Wallis ANOVA, P = 0.0164; Fig. 1a). Circulating PBMC from initial CDI patients contained greater numbers of large and granular cells falling outside gate 1 in gate 2 (Fig. 1b).
Fig. 1.
(a) Total number of cellular events from isolated circulating PBMC needed to obtain 20 000 events in gate 1, standard lymphocyte gate. The circle and asterisk are outlier values. The asterisk represents an outlier value, which lays outside the box at a distance of 1.5x the box size. The circle represents an outlier value greater than the distance of 3x the box size. (b) Total number of cellular events in gate 2, monocyte gate. Error bars represent the SEM.
Circulating CD3+ and CD3− populations in gate 1
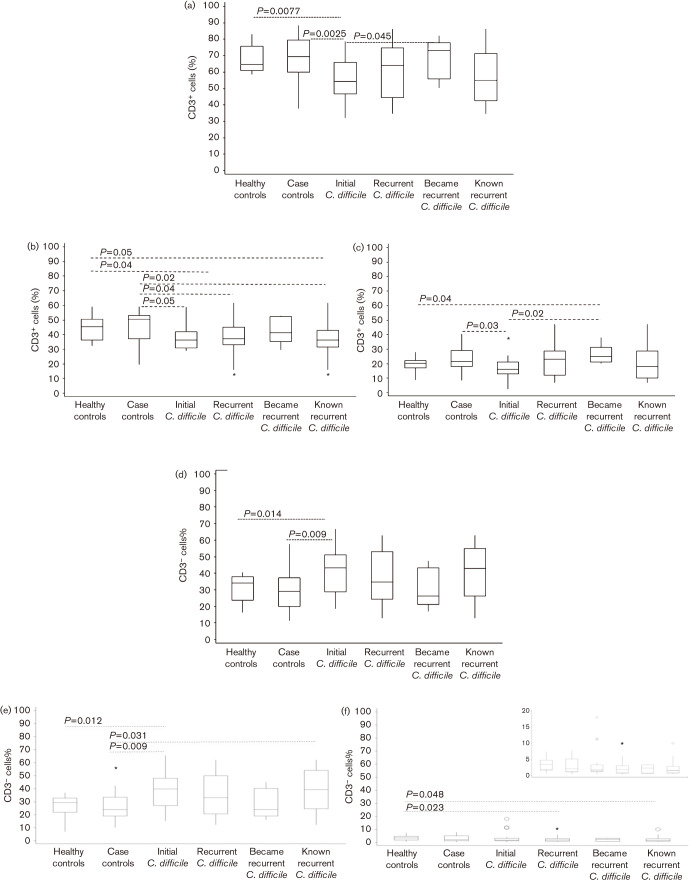
Circulating PMBC from initial CDI patients had a significantly lower percentage of CD3+ lymphocytes when compared with healthy controls (P = 0.0077), case controls (P = 0.0025) and those patients who became recurrent (P = 0.045) (Fig. 2a). Known recurrent CDI patients did not show this significant difference. A ‘box and whisker’ representation of the percentage of CD3+ and CD3− lymphocyte subsets showed broader ranges in the recurrent CDI group when compared with initial CDI, case or healthy controls. Therefore, we decided to subdivide the recurrent population into two smaller subsets because the ‘became recurrent’ CDI subset was distinct. Although this subgroup was very small, it did present us with the opportunity to study phenotypic immune differences at the point of initial infection. We acknowledge that a larger study is needed before any clinical significance can be shown.
Fig. 2.
Ficoll-purified PBMC were stained for CD3 and CD8, then gated on (a) CD3+ or (d) CD3− cells and then analysed for percentage of CD3+ and CD3− cells along with (b) CD3+CD4+, (c) CD3+CD8+, (e) CD3−CD8− and (f) CD3−CD8+ lymphocytes in gate 1. CD3+CD4+ cells were represented by the CD3+CD8− subset in the CD3+ lymphocyte gate. Statistical analysis was carried out using the non-parametric Wilcoxon rank sum test. Circles and asterisks are outlier values. All asterisks represent outlier values, which lay outside the box at a distance of 1.5x the box size. All circles represent outlier values greater than the distance of 3x the box size.
CD3+ cells were further subdivided into CD8+ (CD3+CD8+; Fig. 2b) and CD8− (CD3+CD4+; Fig. 2c) populations. Healthy and case controls had significantly more circulating CD3+CD4+ lymphocytes when compared to all groups that were C. difficile positive. CDI patients had fewer CD3+CD4+ PBMC (Fig. 2b). However, CDI patients who became recurrent had significantly more circulating CD3+CD8+ PBMC when compared with the healthy controls (P = 0.04) and initial CDI groups (P = 0.02; Fig. 2c). The increased CD3+CD8+ circulating PMBC suggested that those CDI patients who became recurrent generated an alternative initial response. The case control group also had significantly greater numbers of CD3+CD8+ lymphocytes when compared with initial CDI patients (P = 0.03; Fig. 2c). As expected, a higher percentage of CD3− lymphocytes circulated in initial CDI patients’ PBMC when compared with healthy (P = 0.014) or case control groups (P = 0.009; Fig. 2d). Most CDI patients had significantly more CD3−CD8− lymphocytes than non-CDI case or healthy controls (Fig. 2e). Healthy controls had more CD3−CD8+ circulating lymphocytes in gate 1 (Fig. 2f).
Pro-inflammatory or cell plasticity phenotype of CD3+CD4+ circulating PMBC in CDI recurrence
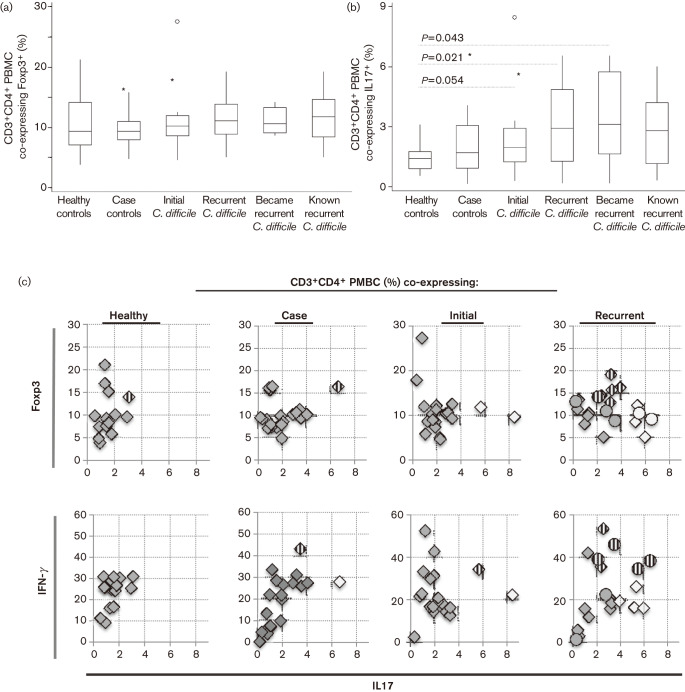
We hypothesized that recurrent CDI patients would have a greater pro-inflammatory T-cell balance represented by increased circulating Th17 lymphocytes. To examine this, we measured the co-expression of Foxp3 (Treg) or IL17 (Th17) on circulating CD3+CD4+ cells. Although recurrent CDI patients had slightly more CD3+CD4+, which co-expressed Foxp3+ (Fig. 3a), we saw no significant difference between groups. Only healthy controls had significantly fewer CD3+CD4+ IL17+ lymphocytes than any patient group (Fig. 3b). While recurrent patients appear to have a broader range and increased median of IL17+-expressing lymphocytes, the difference did not reach significance. To better demonstrate an individual’s overall inflammatory (more IL17 or IFN-γ) or regulatory (Foxp3) CD3+CD4+ phenotype, we compared co-expression of either Foxp3 or IFN-γ against IL-17 for each individual. The IFN-γ vs IL17 plot (Fig. 3c, lower half) showed 50 % of recurrent CDI patients had either a pro-inflammatory CD3+CD4+ phenotype, with increased IFN-γ and IL17 expression (striped circles or diamonds), or predominant IL17 expression (open diamonds). Four other recurrent CDI patients, who did not have increased IFN-γ/IL17 phenotype, had CD3+CD4+ phenotypes with higher Foxp3 and IL17 (striped diamonds, upper plot in Fig. 3c). Taken together, circulating CD3+CD4+ cells from recurrent CDI patients were either ‘plastic’ or predominantly pro-inflammatory. This qualitative difference in the circulating PBMC suggested an alternative immune response during recurrent CDI, one that is skewed towards immune plasticity or inflammation. CDI patients who cleared initial infection had circulating CD3+CD4+ lymphocytes more robustly skewed towards Foxp3, Th17 or IFN-γ, with less plasticity in the T-cell response.
Fig. 3.
PBMC were stained for CD3, CD8 and one of the following: Foxp3, IL17 and IFN-γ. Box and whisker plots showing the range of CD3+CD4+ PBMC which co-express (a) Foxp3 and (b) IL17 in each group. Circles and asterisks are outlier values. All asterisks represent outlier values, which lay outside the box at a distance of 1.5x the box size. All circles represent outlier values greater than the distance of 3x the box size. (c). The percentage of CD3+CD4+ cells which co-expressed IL17 and Foxp3 or IFN-γ was plotted in order to examine the pro-inflammatory or regulatory nature of the CD3+CD4+ population for each individual. Circles represent the six patients whose blood was taken at the time of primary CDI, but who later became recurrent. Striped circles or diamonds represent plasticity of co-expression, and white diamonds or circles represent skewed IL17 co-expression.
Circulating IFN-γ expression
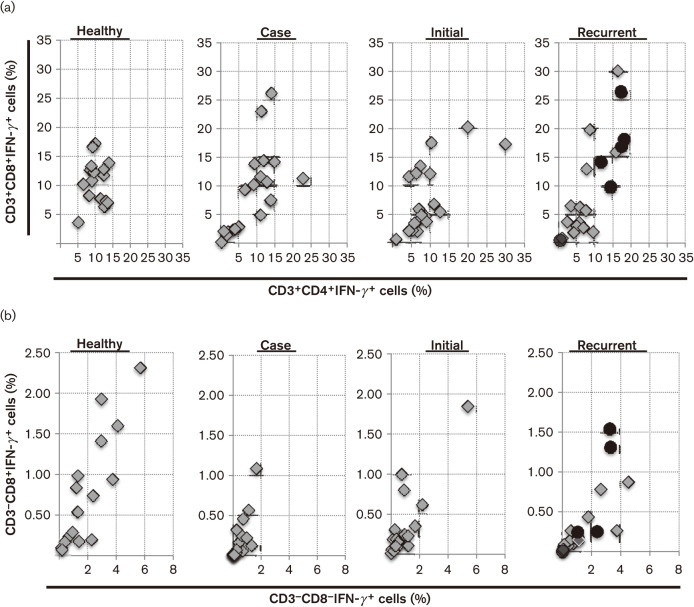
IFN-γ is produced by many different cell types in the innate and adaptive immune response and is a key pro-inflammatory signal (Farrar & Schreiber, 1993; Ishida et al., 2004; Thaiss et al., 2014). We observed IFN-γ expression in CD3+CD4+ (Th1), CD3+CD8+ cytotoxic T-lymphocyte (CTL), natural killer T (NKT)], CD3−CD8+(NK) and CD3−CD8− (macrophages, NK) populations. We examined the CD3+IFN-γ+ phenotype for each individual (Fig. 4a). Two things became apparent: all CD3+CD4+ and CD3+CD8+ circulating lymphocytes from healthy persons demonstrated IFN-γ expression while all hospital patients, either CDI-positive or -negative patients, showed broader ranges of IFN-γ expression. This included no expression of IFN-γ on CD3+ lymphocytes. Circulating CD3+CD4+ lymphocytes from healthy controls (median of 9.85 %) expressed significantly more IFN-γ+ than either initial (P = 0.057, median of 7.43 %) or known recurrent CDI (P = 0.012, median of 6.39 %) patients (box and whisker data not shown). PBMC from healthy persons also contained significantly more CD3+CD8+IFN-γ+ cells (median of 11. 25 %) than known recurrent CDI patients (P = 0.044, median of 4.64 %, grey diamonds). Those patients who became recurrent had more CD3+CD4+IFN-γ+ cells (median of 15.52 %, black circles) than the known recurrent group (P = 0.05, median of 4.64 %) (box and whisker data not shown).
Fig. 4.
Determination of IFN-γ expression in gate 1 PBMC. (a) CD3+ data from each individual plotted as the percentage of CD3+CD4+IFN-γ+ vs CD3+CD8+IFN-γ+ PBMC in gate 1. Black circles in the recurrent subset of patients represent the six patients whose blood was taken at the time of primary CDI, but who later became recurrent. (b) CD3− data from each individual plotted as the percentage of CD3−CD8−IFN-γ+ vs CD3−CD8+IFN-γ+ PBMC in gate 1. Black circles represent the six patients whose blood was taken at the time of primary CDI, but who later became recurrent.
Less IFN-γ co-expression was found on CD3− cells. Although healthy controls had fewer circulating CD3−CD8− lymphocytes (Fig. 2e), they had significantly greater expression of IFN-γ on their circulating CD3− lymphocytes when compared with case control (P = 0.0005, median 0.24 %) and initial CDI (P = 0.016) groups. Recurrent patients showed increased IFN-γ co-expression on both CD3−CD8+ and CD3−CD8− PBMC when compared with initial CDI and case control groups (Fig. 4b). Known recurrent CDI, initial CDI and case control groups all had significantly less IFN-γ expression on CD3−CD8+ cells when compared with healthy controls (P = 0.008, 0.047 and 0.0005, respectively). The case control group of patients had the lowest expression of IFN-γ+ in their CD3− PBMC.
The central role of IFN-γ in the surveillance and protection of a host is demonstrated by the presence of four different IFN-γ+ PBMC populations in the healthy control group. The lack or increase of circulating IFN-γ-co-expressing lymphocytes could be indicative of how the host responds to a pathogen. In this study, five of six CDI patients who became recurrent (black circles in Fig. 4) had increased IFN-γ-co-expressing lymphocytes, indicating a pro-inflammatory (Th1) response at the time of their primary infection.
Foxp3 expression in circulating CD3− lymphocytes
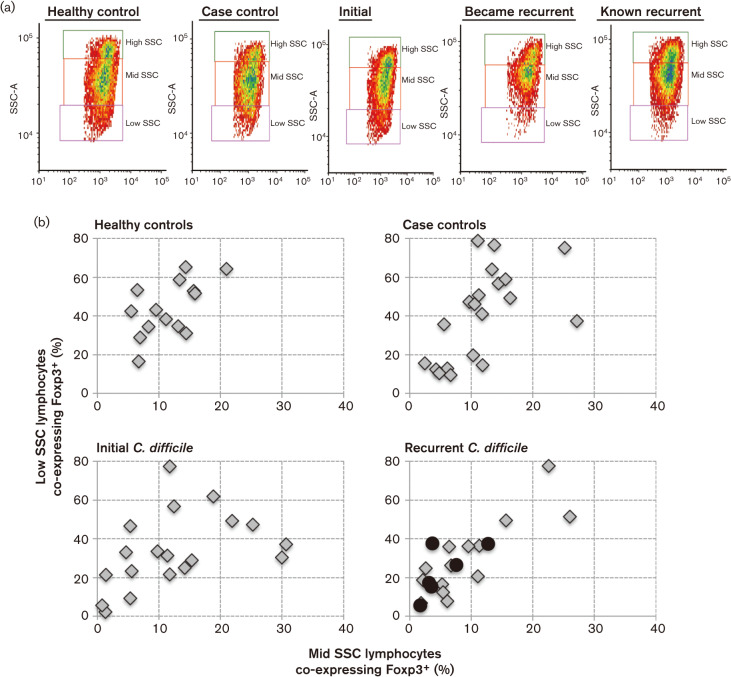
CD3+CD4+Foxp3+ cells were clearly visible upon flow cytometry analysis and there was no significant difference between groups (Fig. 3a). However, upon further analysis, we found the CD3− population also expressed Foxp3. The CD3− subset contained several distinct granular populations (based on SSC); therefore, it was further separated into three subsets, high, middle and low (Fig. 5a). Higher background fluorescence was seen as CD3− cell granularity increased and was accounted for in analysis of each population. The most significant difference in CD3−Foxp3+ population between groups was found in the low SSC CD3− subset. This subset was noticeably diminished in recurrent CDI patients (Fig. 5a). Those CDI patients who became recurrent (median CD3− co-expressing Foxp3+ low SSC 2.03 %) were most significantly (P = 0.0083) different when compared with the initial CDI patient group (median CD3− co-expressing Foxp3+ low SSC 5.76 %) (box and whiskers data not shown). The mid and low SSC CD3− subsets contained the most Foxp3+ co-expression. We compared the Foxp3+ co-expression of low SSC to mid SSC for each individual (Fig. 5b). Many control individuals, either healthy or case, had greater co-expression of Foxp3+ on their CD3− cells (Fig. 5b). Recurrent CDI patients had the least (Fig. 5b). The quantitative and qualitative differences seen in the CD3− lymphocytes continue to demonstrate regulatory distinctions between initial CDI patients and those who become recurrent.
Fig. 5.
Gate 1 PBMC were gated on CD3− cells and plotted showing SSC (representative of the granularity of a cell). (a) Representative flow cytometry plots gated on CD3− lymphocytes and SSC for each group. Recurrent groups have decreased cellular events in low SSC box. (b) The CD3− cells were further gated on high SSC, mid SSC and low SSC. The percentage of cells co-expressing Foxp3 in each of these subsets was calculated. The percentage of mid and low SSC subsets of PBMC co-expressing Foxp3 was plotted for each individual, demonstrating lower Foxp3 expression in the recurrent group. Black circles represent the six patients whose blood was taken at the time of primary CDI, but who later became recurrent.
Pro-inflammatory/regulatory nature of circulating PBMC in CDI
Our original goal was to study equal numbers of circulating lymphocytes from each group. To this end, we collected and analysed a consistent 20 000 events for sample. Our results demonstrated distinct inflammatory or regulatory patterns for circulating CD3− and CD3+ lymphocytes from each group. However, circulating PBMC from initial CDI patients contained more cells that fell outside the lymphocyte gate (gate 1) into the monocyte gate (gate 2). Therefore, to better understand immune balance in control and CDI groups, we compared inflammatory (IFN-γ+IL17) and regulatory (Foxp3) expression in each gate as a percentage of total events collected. Results are shown in Fig. 6 and Table 4. Infection with C. difficile changed the phenotypic balance of circulating cells when compared with both healthy and case controls (Fig. 6a–c). PBMC from initial CDI patients express more Foxp3 on cells in gate 2 when compared to all other groups, including the CDI recurrent patients (Fig. 6b). Comparisons of pro-inflammatory subsets were determined by correlating IL17 and IFN-γ expression together or separately with Foxp3 expression. Results are shown in Table 4. IL17 and Foxp3 expression in gate 1 was positively correlated in healthy and case controls as well as recurrent CDI groups (0.3231, 0.6014, 0.4331, respectively). The positive correlation rose to significance in the case control group (0.0058), and was close to significance in the recurrent group (0.0569). A negative and non-significant correlation characterized the Foxp3 vs IL17 gate 1 lymphocytes from initial CDI patients (−0.1440).
Fig. 6.
Regulatory (Foxp3 expression) vs inflammatory (IL17 and IFN-γ of all circulating PBMC. Gates 1+2 represent over 85 % of cells collected in each group. Up to 15 % of the cellevents collected resided outside of these gates, but formed no other major subset of cells. The percentage of all populations (CD3+ and CD3−) expressing Foxp3 or IL17 and IFN-γ from (a) gate 1 – lymphocytes, (b) gate 2 – monocytes or larger cells, (c) overall PBMC gates 1 and 2. Black circles represent the six patients whose blood was taken at the time of primary CDI, but who later became recurrent.
Table 4.
Pro-inflammatory/regulatory nature of circulating PBMC in gates 1 and 2
The percentage of each marker (IL17, Foxp3 or IFN-γ) was calculated as a percentage of total PBMC events collected. Gate 1 represents the cells found in the standard lymphocyte gate. Gate 2 represents the cells found in the standard monocyte gate. Gates 1+2 represents over 85 % of cells collected in each group. Up to 15 % of the cell events collected resided outside of these gates, but formed no other major subset of cells. Spearman rank correlations were used to compare the relationship between pro-inflammatory and regulatory markers in the PBMC.
| Patient group | Gate | Spearman rank correlation (significant P value) | |||
| Foxp3 vs IL17 & IFN-γ | Foxp3 vs IL17 | Foxp3 vs IFN-γ | IFN-γ vs IL17 | ||
| Healthy controls | Gate 1 | 0.1562 | 0.3231 | 0.1033 | 0.3934 |
| Gate 2 | 0.2310 | −0.0066 | 0.2948 | 0.5363 (0.05) | |
| Gate 1+2 | 0.2222 | 0.0264 | 0.2222 | 0.3099 | |
| Case controls | Gate 1 | 0.6386 (0.0030) | 0.6014 (0.0058) | 0.6356 (0.0032) | 0.8397 (0.0000) |
| Gate 2 | 0.5198 (0.0238) | 0.4119 | 0.5749 (0.0113) | 0.8639 (0.0000) | |
| Gate 1+2 | 0.6772 (0.0019) | 0.4958 (0.0323) | 0.7070 (0.0010) | 0.8065 (0.0000) | |
| Initial C. difficile | Gate 1 | 0.0246 | −0.1440 | 0.0561 | 0.2284 |
| Gate 2 | 0.4432 (0.0585) | 0.4451 (0.0574) | 0.5099 (0.0272) | 0.7582 (0.0002) | |
| Gate 1+2 | 0.0193 | 0.0298 | 0.0281 | 0.2439 | |
| Recurrent C. difficile | Gate 1 | 0.4226 | 0.4331 (0.0569) | 0.4241 | 0.5970 (0.0062) |
| Gate 2 | 0.4414 (0.0524) | 0.2102 | 0.4369 (0.0551) | 0.8195 (0.0000) | |
| Gate 1+2 | 0.4692 (0.0378) | 0.2932 | 0.4752 (0.0351) | 0.6346 (0.0032) | |
Discussion
CDI and its recurrence is a mounting healthcare problem. Understanding host immune responses to this infection will be key to development of non-antibiotic therapies (CDC, 2013; Foglia et al., 2012; Lo Vecchio & Zacur, 2012; Lowy et al., 2010; van Nood et al., 2013; Villano et al., 2012). Most current therapeutic focus revolves around the humoral response. Focus is on boosting the humoral response to toxins, removal of toxins using anti-toxin specific antibodies or altered colonization (Foglia et al., 2012; Lo Vecchio & Zacur, 2012; Lowy et al., 2010; van Nood et al., 2013; Villano et al., 2012). However, the phenotype of the cellular immune response necessary for clearance and protection in CDI patients is less defined (Monaghan et al., 2013). Cellular responses to pathogenic invasion, including CDI, involve both the innate and adaptive immune cells and have been studied using PBMC from healthy persons, but not from CDI patients (Ausiello et al., 2006; Bianco et al., 2011; Jafari et al., 2013; Koon et al., 2013; Mahida et al., 1998; Wu et al., 2013). We examined cellular immune differences between those who productively clear CDI and those who become recurrent. This work complements others that have focused on gut bacterial ecology, colonization and the humoral immune response as factors resulting in CDI (Adlerberth et al., 2014; Martin et al., 2013; Péchiné et al., 2007; Rousseau et al., 2012; Villano et al., 2012). We examined the balance of Th1, Treg and Th17 cells in PBMC and are the first, to our knowledge, to demonstrate quantitative and qualitative differences in circulating lymphocytes from those that clear CDI (initial CDI), recurrent CDI cases, comorbid CDI negative case controls and healthy controls.
Using peripheral blood to study systemic effects of local disease has given insights into both the humoral and cellular processes involved in autoimmune and chronic inflammatory diseases (Malmhäll et al., 2012; Monaghan et al., 2013; Omoyinmi et al., 2012; Ueno et al., 2013). Definition of target populations, timing of sample procurement, disease course and severity, age and comorbidities all impact data analysis and comparisons between studies. For example, Bulusu et al. (2000) showed white blood cell WBC counts fluctuate temporally during CDI, and three general patterns of disease course could be discerned. His cohort was male dominated, over 60 years of age, and many had cancer (Bulusu et al., 2000). Lavergne et al. (2013) reported the presence of lymphopenia at the end of SOC treatment for CDI. These authors suggested that the initial T-cell response was not robust and the lymphopenia could be used as a transient biomarker for recurrence (Lavergne et al., 2013). Forty-two per cent of this population was female and the mean age was 77, suggesting that age and immune senescence could also be involved. In our own analysis, we ended up with two distinct recurrent populations. Both represented the standard definition of recurrence but the time of PBMC collection and consent were different. Although this confounded interpretation, subdividing the recurrent group into smaller subgroups for further analysis presented an opportunity for insight into how the immune response differs at the point of primary CDI as well as recurrence. Further study with larger accrual of this key population (primary onset but become recurrent) is needed to determine if this is of clinical significance.
Our primary goal was to examine cellular responses, in particular Th1, Th17 and Treg, and we acknowledge that we excluded up to 50 % of CDI patients with cancer, immunosuppression and inflammatory bowel disease. Excluding these patients provided groups more likely to have more similar circulating T-cell populations. Our demographics, which included non-CDI comorbid patients, indicated we had accrued slightly more females, with mean ages younger than many reported studies. Our population’s trend toward younger age is a reflection of being an urban tertiary care centre with more complicated patients. Furthermore, increasing CDI is found in younger populations in the community. Therefore, our patient cohort provided us the opportunity to study a host’s T-cell phenotype and how it represents a productive cellular immune response to CDI in our current urban environment.
Our CDI patients presented with a WBC range of 5.6 to 24.5×103 μl−1, but the composition of isolated PBMC was more indicative of host response to CDI. The circulating PBMC from those who ‘became recurrent’ was different from that of those patients known to be recurrent. Specifically, increased CD3+ PBMC marked the initial CDI response of six patients who became recurrent, and the percentage of CD3+CD8+, not CD3+CD4+, was significantly different (Fig. 2).
Increased expression of circulating proinflammatory TH17 CD3+ cells is seen in several immune disorders, including asthma, multiple sclerosis and inflammatory bowel disease (Malmhäll et al., 2012; Ueno et al., 2013). Furthermore, it has recently been demonstrated that pre-exposure to Th17-inducing adjuvant intensifies mucosal inflammation after viral infection (Gopal et al., 2014). Hence, the nature of the initial cellular CDI response and subsequent intestinal or systemic environment will be important to clearance and non-recurrence. Work by El Feghaly et al. (2013a, b) showed markers of intestinal inflammation, not C. difficile burden, predicated outcome of CDI and demonstrated the importance of the inflammatory intestinal environment in CDI clearance. Our study builds on these observations and demonstrates a greater pro-inflammatory balance also exists in circulating PBMC in our recurrent CDI patients. Although the Th17 cell type was originally thought of as a separate lineage, this phenotype can shift towards Th1 or Th2, intensifying the biological process (Cosmi et al., 2014). In CDI, increased IFN-γ production may sustain the inflammatory process. Our data show that the early cellular immune response in those who became recurrent was composed of ≥30 % circulating PBMC co-expressing IFN-γ, while ≤20 % of circulating PMBC co-expressed IFN-γ in patients who cleared CDI or were already known to be recurrent. Our data are the first, to our knowledge, to demonstrate the importance of early cellular responses to CDI and how the phenotype of CDI patients changes over the course of the infection.
Some of the recurrent patients showed increased IL17/IFN-γ or IL17/Foxp3 phenotypes. Although expression of Foxp3, IL17 and IFN-γ was examined in separate wells, we cannot rule out that some circulating PMBC co-express either IL17 and IFN-γ or IL17 and Foxp3. The concept of T-cell plasticity extends back 20 years with murine data and cell line studies (Malmhäll et al., 2012). Malmhäll et al. (2012) described T-cell plasticity in asthma as well as healthy controls, suggesting two arms describe plasticity. Plastic cells either are not committed or are characterized by multiple transcription factors (Malmhäll et al., 2012). Recently, a novel IL17-secreting CD4+ cell co-expressing Foxp3 has been described in inflammatory bowel disease (Ueno et al., 2013). Functionally, the presence of this cell type appears to decrease ability of Treg to suppress T-cell proliferation. Our data indirectly demonstrate that recurrent patients’ circulating CD3+CD4+ cells can be either skewed towards a Foxp3/IL17, IL17/IFN-γ phenotype or driven towards a Th17 phenotype. The circulating PBMC phenotype from initial CDI patients appears driven towards committed end points.
Expression of Foxp3, both on CD3+ and CD3− cells, appears in the phenotype of all groups. The phenotypic difference between CDI clearance and recurrence is decreased co-expression of Foxp3 on the CD3− cells. A decrease is also seen in the number of circulating CD3− low SSC cells. The cell type remains unidentified, but due to lower granularity it could possibly be a B-cell. Circulating CD5+CD19+Foxp3+ cells have been described, but no function has been attributed to this cell type. Other regulatory B cells have been associated with IL-10 and transforming growth factor (TGF)-β production and induction of Treg (Berthelot et al., 2013; Noh et al., 2010; Vadasz et al., 2013). Further studies need to be done to identify the CD3−Foxp3+ population and determine its potential role in CDI clearance and recurrence.
Our study demonstrated phenotypic differences between CDI-positive and -negative patients, specifically within the CD3+ and CD3− populations. Plastic and pro-inflammatory (Foxp3/IL17, IFN-γ/IL17, IL17 skewed) CD3+CD4+ phenotypes typified most CDI recurrent patients when compared with initial CDI, case or healthy control groups. Furthermore, at time of primary onset of CDI, those patients who clear initial infection and those patients who go on to become recurrent are phenotypically divergent. This small subgroup presented with circulating PBMC skewed towards a pro-inflammatory adaptive response with increased IFN-γ, IL17 and CD3+CD4+ plasticity. The immune response to CDI is not static. Many of these PBMC phenotypic differences become less distinguishable in the known recurrent CDI subgroup. Successful CDI clearance was characterized by circulating PBMC composed of more CD3− lymphocytes, greater numbers of cells which fall in the standard monocyte gate, a less plastic CD3+CD4+ response, fewer IFN-γ-co-expressing lymphocytes and greater numbers of Foxp3+-co-expressing CD3− lymphocytes. Our pilot study further demonstrates the role of cellular inflammation in recurrent CDI and suggests the need for better patient stratification and alternate therapeutic choices, which may include combination therapy.
Acknowledgements
This work was supported by a Merck Sharp & Dohme Corporation Investigator Initiated study (IISP) proposal grant (38933) to B. Y. We thank Dr Madhuri Sopirala MD, UC division of Infectious Disease and Medical Director Department of Infection Control and Antimicrobial Stewardship UC Health, and Ms Carol Hamburg, UC Health Center infection preventionist, for their help in identifying and referring CDI patients; Dr David Bernstein MD and Dr Deb Ghosh PhD for the guidance and use of their flow cytometer.
Footnotes
Abbreviations: CDI, Clostridium difficile infection; PBMC, peripheral blood mononuclear cell(s); Treg, T regulatory; SOC, standard of care; SSC, side scatter; WBC, white blood cell.
References
- Adlerberth I., Huang H., Lindberg E., Åberg N., Hesselmar B., Saalman R., Nord C. E., Wold A. E., Weintraub A. Toxin-producing Clostridium difficile strains as long-term gut colonizers in healthy infants. J Clin Microbiol. 2014;52:173–179. doi: 10.1128/JCM.01701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T.& other authors (2011Induction of colonic regulatory T cells by indigenous Clostridium species Science 331337–341. 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausiello C. M., Cerquetti M., Fedele G., Spensieri F., Palazzo R., Nasso M., Frezza S., Mastrantonio P. Surface layer proteins from Clostridium difficile induce inflammatory and regulatory cytokines in human monocytes and dendritic cells. Microbes Infect. 2006;8:2640–2646. doi: 10.1016/j.micinf.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Berthelot J. M., Jamin C., Amrouche K., Le Goff B., Maugars Y., Youinou P. Regulatory B cells play a key role in immune system balance. Joint Bone Spine. 2013;80:18–22. doi: 10.1016/j.jbspin.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Bianco M., Fedele G., Quattrini A., Spigaglia P., Barbanti F., Mastrantonio P., Ausiello C. M. Immunomodulatory activities of surface-layer proteins obtained from epidemic and hypervirulent Clostridium difficile strains. J Med Microbiol. 2011;60:1162–1167. doi: 10.1099/jmm.0.029694-0. [DOI] [PubMed] [Google Scholar]
- Bulusu M., Narayan S., Shetler K., Triadafilopoulos G. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am J Gastroenterol. 2000;95:3137–3141. doi: 10.1111/j.1572-0241.2000.03284.x. [DOI] [PubMed] [Google Scholar]
- CDC. Antibiotic resistance threats in the United States. 2013. http://www.cdc.gov/drugresistance/threat-report-2013 Available at. [accessed 3 December 2013] [PubMed]
- Chiba T., Seno H. Indigenous Clostridium species regulate systemic immune responses by induction of colonic regulatory T cells. Gastroenterology. 2011;141:1114–1116. doi: 10.1053/j.gastro.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Cosmi L., Maggi L., Santarlasci V., Liotta F., Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014;85:36–42. doi: 10.1002/cyto.a.22348. [DOI] [PubMed] [Google Scholar]
- El Feghaly R. E., Stauber J. L., Deych E., Gonzalez C., Tarr P. I., Haslam D. B. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis. 2013a;56:1713–1721. doi: 10.1093/cid/cit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Feghaly R. E., Stauber J. L., Tarr P. I., Haslam D. B. Intestinal inflammatory biomarkers and outcome in pediatric Clostridium difficile infections. J Pediatr. 2013b;163:1697–1704, e2. doi: 10.1016/j.jpeds.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Foglia G., Shah S., Luxemburger C., Pietrobon P. J. F. Clostridium difficile: development of a novel candidate vaccine. Vaccine. 2012;30:4307–4309. doi: 10.1016/j.vaccine.2012.01.056. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V., Rakotobe S., Lécuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M.& other authors (2009The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses Immunity 31677–689. 10.1016/j.immuni.2009.08.020 [DOI] [PubMed] [Google Scholar]
- Gopal R., Rangel-Moreno J., Fallert Junecko B. A., Mallon D. J., Chen K., Pociask D. A., Connell T. D., Reinhart T. A., Alcorn J. F.& other authors (2014Mucosal pre-exposure to Th17-inducing adjuvants exacerbates pathology after influenza infection Am J Pathol 18455–63. 10.1016/j.ajpath.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Maegawa T., Kondo T., Kimura A., Iwakura Y., Nakamura S., Mukaida N. Essential involvement of IFN-γ in Clostridium difficile toxin A-induced enteritis. J Immunol. 2004;172:3018–3025. doi: 10.4049/jimmunol.172.5.3018. [DOI] [PubMed] [Google Scholar]
- Jafari N. V., Kuehne S. A., Bryant C. E., Elawad M., Wren B. W., Minton N. P., Allan E., Bajaj-Elliott M. Clostridium difficile modulates host innate immunity via toxin-independent and dependent mechanism(s) PLoS ONE. 2013;8:e69846. doi: 10.1371/journal.pone.0069846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C. P. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(Suppl. 6):21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- Kelly C. P., Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070–1079. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- Koon H. W., Shih D. Q., Hing T. C., Yoo J. H., Ho S., Chen X., Kelly C. P., Targan S. R., Pothoulakis C. Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes. Antimicrob Agents Chemother. 2013;57:3214–3223. doi: 10.1128/AAC.02633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne V., Beauséjour Y., Pichette G., Ghannoum M., Su S. H. Lymphopenia as a novel marker of Clostridium difficile infection recurrence. J Infect. 2013;66:129–135. doi: 10.1016/j.jinf.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Lo Vecchio A., Zacur G. M. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28:1–9. doi: 10.1097/MOG.0b013e32834bc9a9. [DOI] [PubMed] [Google Scholar]
- Lowy I., Molrine D. C., Leav B. A., Blair B. M., Baxter R., Gerding D. N., Nichol G., Thomas W. D., Jr, Leney M.& other authors (2010Treatment with monoclonal antibodies against Clostridium difficile toxins N Engl J Med 362197–205. 10.1056/NEJMoa0907635 [DOI] [PubMed] [Google Scholar]
- Madan R., Petri W. A., Jr Immune responses to Clostridium difficile infection. Trends Mol Med. 2012;18:658–666. doi: 10.1016/j.molmed.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Galvin A., Makh S., Hyde S., Sanfilippo L., Borriello S. P., Sewell H. F. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect Immun. 1998;66:5462–5469. doi: 10.1128/iai.66.11.5462-5469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmhäll C., Bossios A., Rådinger M., Sjöstrand M., Lu Y., Lundbäck B., Lötvall J. Immunophenotyping of circulating T helper cells argues for multiple functions and plasticity of T cells in vivo in humans possible role in asthma. PLoS ONE. 2012;7:e40012. doi: 10.1371/journal.pone.0040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. H., Chen Z. M., Tang Y. M., Liang L., Du L. Z., Zhang Y. [Regulation of CD3, CD4 and CD8 expressions on PMA-activated human peripheral T cells] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2004;33:155–159. doi: 10.3785/j.issn.1008-9292.2004.02.015. (in Chinese) [DOI] [PubMed] [Google Scholar]
- Martin J., Mawer D., Wilcox M. H. Clostridium difficile: biological therapies. Curr Opin Infect Dis. 2013;26:454–460. doi: 10.1097/01.qco.0000433319.82618.8f. [DOI] [PubMed] [Google Scholar]
- Monaghan T. M., Robins A., Knox A., Sewell H. F., Mahida Y. R. Circulating antibody and memory B-cell responses to C. difficile toxins A and B in patients with C. difficile-associated diarrhoea, inflammatory bowel disease and cystic fibrosis. PLoS ONE. 2013;8:e74452. doi: 10.1371/journal.pone.0074452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J., Choi W. S., Noh G., Lee J. H. Presence of Foxp3-expressing CD19(+)CD5(+) B cells in human peripheral blood mononuclear cells: human CD19(+)CD5(+)Foxp3(+) regulatory B cell (Breg) Immune Netw. 2010;10:247–249. doi: 10.4110/in.2010.10.6.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoyinmi E., Hamaoui R., Pesenacker A., Nistala K., Moncrieffe H., Ursu S., Wedderburn L. R., Woo P. Th1 and Th17 cell subpopulations are enriched in the peripheral blood of patients with systemic juvenile idiopathic arthritis. Rheumatology (Oxford) 2012;51:1881–1886. doi: 10.1093/rheumatology/kes162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péchiné S., Janoir C., Boureau H., Gleizes A., Tsapis N., Hoys S., Fattal E., Collignon A. Diminished intestinal colonization by Clostridium difficile and immune response in mice after mucosal immunization with surface proteins of Clostridium difficile. Vaccine. 2007;25:3946–3954. doi: 10.1016/j.vaccine.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Parsons I. J., Marsh M. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J Exp Med. 1993;178:1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planche T. Clostridium difficile. Medicine. 2013;41:654–657. doi: 10.1016/j.mpmed.2013.08.003. [DOI] [Google Scholar]
- Rousseau C., Poilane I., De Pontual L., Maherault A. C., Le Monnier A., Collignon A. Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin Infect Dis. 2012;55:1209–1215. doi: 10.1093/cid/cis637. [DOI] [PubMed] [Google Scholar]
- Spellberg B., Guidos R., Gilbert D., Bradley J., Boucher H. W., Scheld W. M., Bartlett J. G., Edwards J., Jr, Infectious Diseases Society of America The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- Thaiss C. A., Levy M., Suez J., Elinav E. The interplay between the innate immune system and the microbiota. Curr Opin Immunol. 2014;26:41–48. doi: 10.1016/j.coi.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Ueno A., Jijon H., Chan R., Ford K., Hirota C., Kaplan G. G., Beck P. L., Iacucci M., Fort Gasia M.& other authors (2013Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients Inflamm Bowel Dis 192522–2534. 10.1097/MIB.0b013e3182a85709 [DOI] [PubMed] [Google Scholar]
- Vadasz Z., Haj T., Kessel A., Toubi E. B-regulatory cells in autoimmunity and immune mediated inflammation. FEBS Lett. 2013;587:2074–2078. doi: 10.1016/j.febslet.2013.05.023. [DOI] [PubMed] [Google Scholar]
- van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E. G., de Vos W. M., Visser C. E., Kuijper E. J., Bartelsman J. F. W. M.& other authors (2013Duodenal infusion of donor feces for recurrent Clostridium difficile N Engl J Med 368407–415. 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- Villano S. A., Seiberling M., Tatarowicz W., Monnot-Chase E., Gerding D. N. Evaluation of an oral suspension of VP20621, spores of nontoxigenic Clostridium difficile strain M3, in healthy subjects. Antimicrob Agents Chemother. 2012;56:5224–5229. doi: 10.1128/AAC.00913-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Joyee A. G., Nandagopal S., Lopez M., Ma X., Berry J., Lin F. Effects of Clostridium difficile toxin A and B on human T lymphocyte migration. Toxins (Basel) 2013;5:926–938. doi: 10.3390/toxins5050926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacyshyn M. B., Yacyshyn B. The role of gut inflammation in recurrent Clostridium difficile-associated disease. Clin Infect Dis. 2013;56:1722–1723. doi: 10.1093/cid/cit151. [DOI] [PubMed] [Google Scholar]