Fig. 5. Remodeling of the free-energy landscape of apo RAB by Rp and the PKA C-subunit explains allosteric pluripotency.
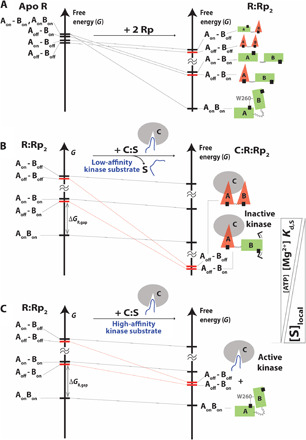
(A) Effect of Rp binding on the ensemble of states accessible to apo RAB. As the Boltzmann populations of the state decrease, the size of the cartoon is reduced. The inhibitory-competent conformations of RAB:Rp2 (red bars) are excited states. (B) Effect of PKA C-subunit binding on the ensemble of states accessible to RAB:Rp2. If the binding of the substrate to the kinase is sufficiently weak, and the MgATP level is sufficiently high, the inhibitory states “Aoff-Boff” and “Aoff-Bon” become the most stable conformations of RAB in the C:RAB:Rp2 ensemble. The kinase is inactive. ΔGR,gap is the inhibitory excited versus noninhibitory ground state free-energy difference. (C) In the case of substrates that bind C with sufficiently high affinity to compete with RAB or when the MgATP level decreases, due to the lower effective affinity of RAB:Rp2 for C, the most stable conformation of RAB accessible to the C:RAB:Rp2 ensemble is the “AonBon” state, which is noninhibitory. Hence, the kinase is active.
