Fig. 6. Changes in MgATP concentration and/or substrate affinity trigger the Rp antagonism-to-agonism switch for PKA R1a.
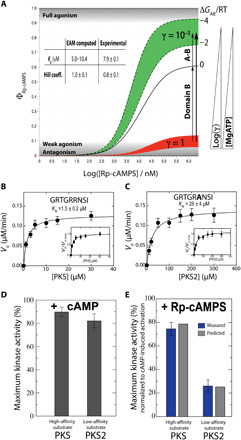
(A) Antagonism-to-agonism switch triggered by [MgATP] reduction. Dose-dependent kinase activation (ϕ) in the presence (red, γ = 1) and absence of MgATP (green, γ = 10−3). The γ scaling factor models the reduction in R:C affinity occurring upon removal of MgATP (table S1). (B to E) Antagonism-to-agonism switch triggered by substrate affinity increases. The substrates PKS and PKS2 exhibit Km values different by approximately one order of magnitude (B and C), and the increase in apparent affinity causes a switch to almost full agonism for Rp-cAMPS (E), while cAMP acts as an activator for both substrates, irrespective of the apparent affinity (D). In (E), blue bars are from kinase assays, while gray bars are computed on the basis of the EAM (see Materials and Methods). Error bars reflect the SD from triplicate measurements.
