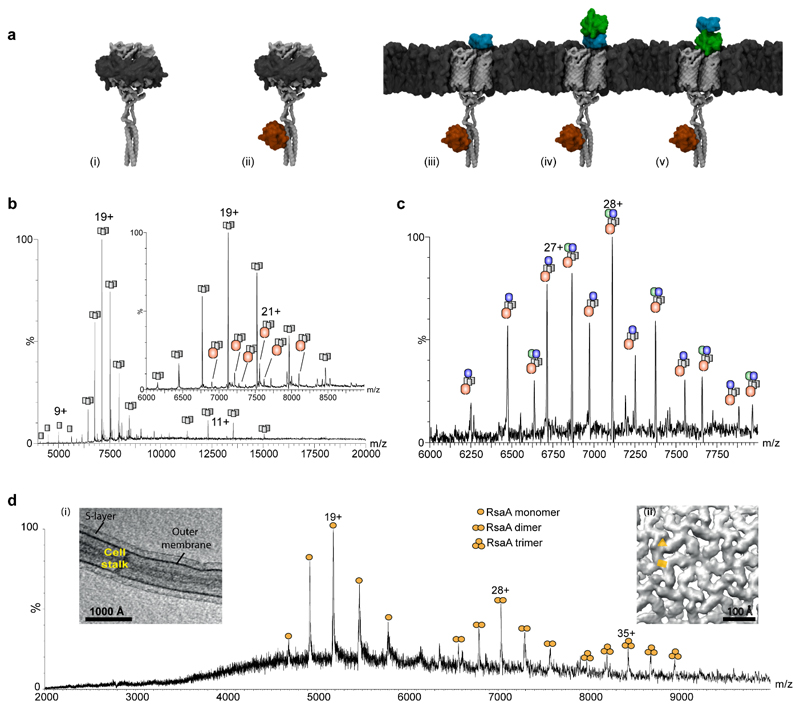
Figure 5. Comparison of SoLVe-MS with low pH and detergent extracted membrane protein complexes and associated proteins.
(a) Models of Fap protein complexes observed by detergent extraction (i)-(ii) and by SoLVe-MS (iii) – (v). The interaction of FapF with FapD is modeled based on BS3 cross-linking and data reported previously45, and models for FapC and E are homology based. (b) Mass spectrum of the Fap operon purified in C8E4 shows predominantly FapF3 (i) (grey) alone, and with a very low population containing a single FapD (ii) (red), or a single misprocessed FapD (red). (c) SoLVe-MS of E. coli membranes lacking major outer membrane components, overexpressing the Fap operon, show association of FapF3 with FapD and FapC (blue) (iii) or FapD, FapC and FapE (green) (iv) or (v). It is not known whether or not FapE (green) displaces FapC (blue), so both models are presented. Spectra were acquired at 400 V (Desolvation -200V, Source fragmentation 200V), and are a representative from 2 biological repeats and multiple MS experiments. (d) SoLVe-MS enables release of RsaA from the surface of the membrane, from the cell stalk. Inset (i) Tomographic slice through a C. crescentus stalk. Inset (ii) Sub-tomogram averaging of the purified cell stalks shows the presence of two-fold and a pseudo-three-fold symmetry axes in the S-layer lattice on native membranes, marked with a yellow rectangle and triangle respectively. Spectrum was recorded at a Desolvation -150 V, source fragmentation 100 V and HCD 150 V.