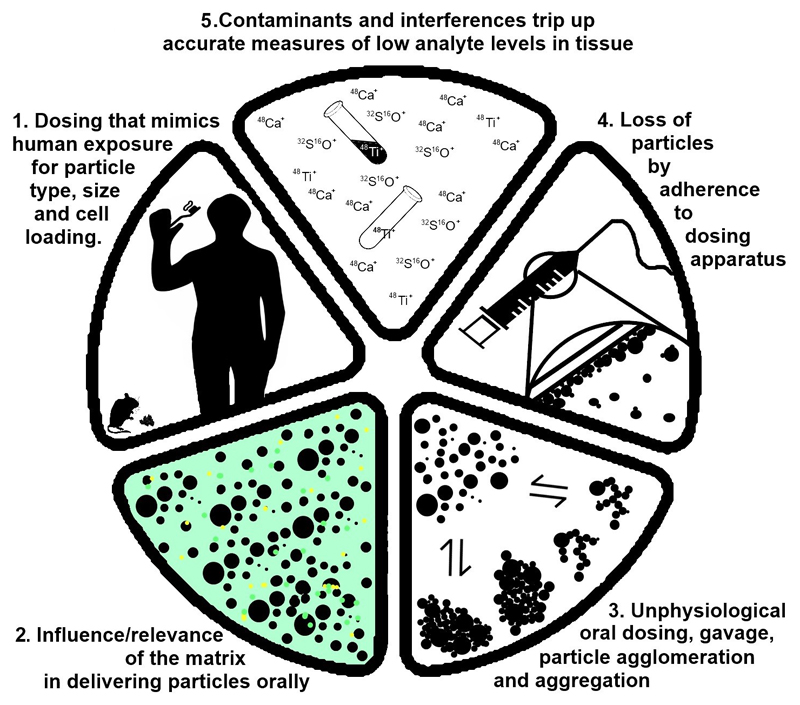
Figure 2. Important considerations and caveats in absorption studies.
Murine models are frequently used to predict effects of oral particle exposure to humans but, mostly, these are flawed. Mimicking particle type is important whilst comparative cell loading (i.e. in the rodent model versus humans) may turn out to be the only reasonable, albeit technically demanding, approach for sensible quantitative dosing (1). If human cell exposure to oral particles is known, as it is for fgTiO2 and some silicates [3], then any dosing matrix needs to allow some gut luminal release of such particles in an animal model (2). Gavage is stressful and its bolus doses are totally unlike general oral exposure to particles for humans. Moreover, such approaches encourage agglomeration and aggregation of particles (3). In addition, particles are sticky and adsorb to such dosing equipment (4). Finally, even if the issues of 1-4 are avoided, then analysis of particles in tissues must be very carefully validated as errors are frequent (5).