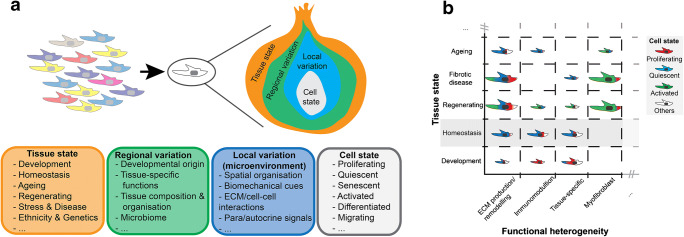
Fig. 1.
Strategies for fibroblast stratification. a Layers of the fibroblast phenotype. Within a tissue, the diversity of the fibroblast population (e.g. as identified by single-cell RNA-seq) will be the combined reflection of the tissue state, regional/anatomical variations, local heterogeneity (microenvironment) and cellular state. b Discovering how different tissue states influence fibroblast heterogeneity. Single-cell RNA-seq generally starts with adult homeostatic tissue to define fibroblast heterogeneity at a local level (e.g. tissue biopsy; highlighted in grey). These efforts have revealed subpopulations with distinct functionality that are predominately in a quiescent state. How these lineages develop is variable between organ systems, but involves significant proliferation of a pool of multipotent progenitors that ultimately differentiate into specialised subsets. Upon tissue injury, fibroblasts become activated and may transiently change their relative abundances and functionality. New subpopulations (e.g. myofibroblast) may appear during this process from one or several precursors. More significant and persistent changes in fibroblast heterogeneity have been observed in fibrotic disease conditions (e.g. accumulation and dominance of continuously active myofibroblasts, which themselves are diverse). With age, fibroblast abundance and diversity decline, which may impair organ function or ability to regenerate. Cell size indicates fibroblast subpopulation abundance and the colouring illustrates different cellular states within a functionally distinct population. schematic provides some illustrative examples only that is based upon current literature but do not represent a specific organ system.