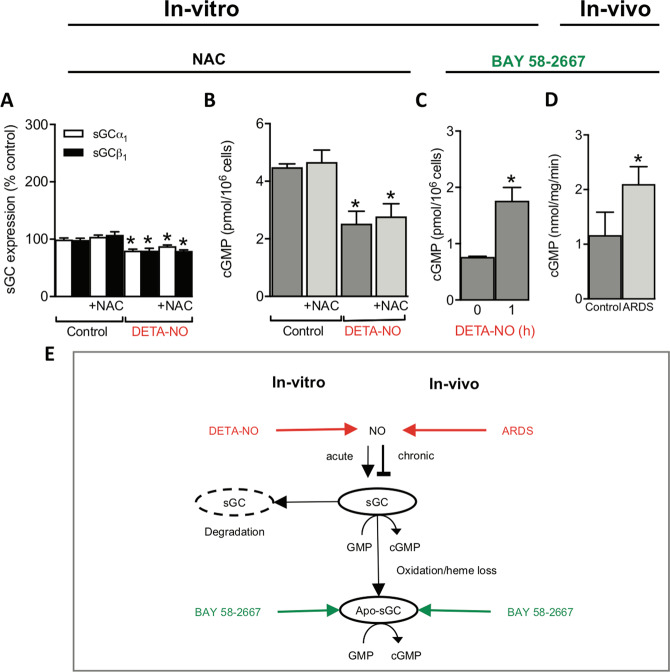
Figure 3.
NO-induced sGC downregulation is thiol-independent but involves sGC loss and a shift towards apo-sGC. When PPAECs were exposed for 72 h to DETA-NO (100 µM) in the absence and presence of N-acetyl-L-cysteine (NAC; 1 mM), NAC neither affected sGC protein levels (N = 5) (A) nor activity (N = 4) (B). Exposure of PPAECs for 72 h to DETA-NO (100 µM) increased apo-sGC activity, measured as BAY 58-2667-induced cGMP formation (BAY 58-2667, 10 µM) (N = 3) (C). Validation of the above in-vitro mechanistic findings in-vivo in the porcine high-NO ARDS model showing also increased apo-sGC activity (N = 3) (D). (E) A scheme summarizing both our in-vitro and in-vivo data that both endogenous NO or pharmacological NO donor compounds that acutely stimulate sGC, chronically decreased both sGC protein and activity leading to inactivation of sGC and an apparent net shift towards NO-insensitive apo-sGC. Data are expressed as mean ± SEM. *,**p < 0.05 or 0.01 vs. control, respectively. Representative full-length blots are presented in Supplementary Figure S4.