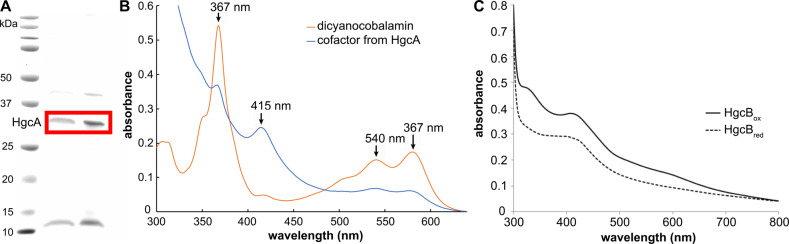
Fig. 1. Protein purification and spectral characterization.
a SDS-PAGE gel of purified HgcA. The bands enclosed in the red rectangle are HgcA in elution buffer and after buffer exchange, respectively, as verified by western blot analysis using an antibody against the His-tag. A full, uncropped gel image is provided in Supplementary Fig. 1. b UV–visible spectrum of dicyanocobalamin (orange) and cofactor extracted from purified, His-tagged HgcA by heating to 95 °C with KCN (blue). HgcA was dissolved in phosphate buffer (50 mM K2HPO4, 100 mM NaCl, 10% glycerol, 2 mM BME, 10 mM imidazole, pH 7.4). c UV–visible spectrum of oxidized, as-isolated MBP-HgcB (HgcBox) and MBP-HgcB after reduction with sodium dithionite (HgcBred).