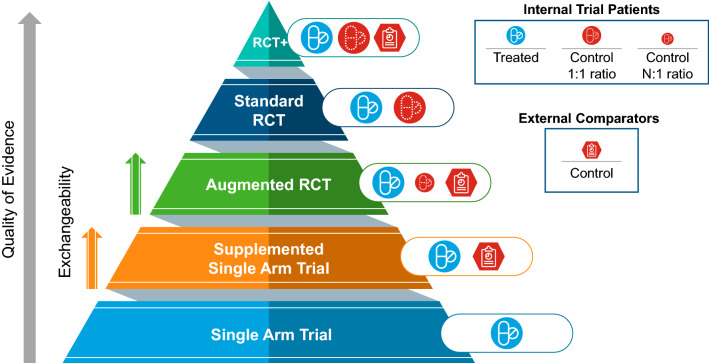
Fig. 1.
Study design hierarchy of evidence. The proposed hierarchy of evidence for study designs in the context of use of an external comparator arm from real-world data in comparison to a standard randomised controlled trial (RCT) or a single-arm trial. The quality of evidence, as indicated by the filled arrow, is expected to increase as one goes from a single-arm trial to an RCT; similarly, within the designs for trials using real-world data external comparators, the quality of evidence is dependent upon exchangeability, as indicated by the striped arrows, as it is expected to increase as the exchangeability status between the trial patients and the external comparators transitions from being poor (non-exchangeable), partially exchangeable or completely exchangeable. RCT+ represents study designs that go above and beyond by having a fully powered RCT complemented by external data