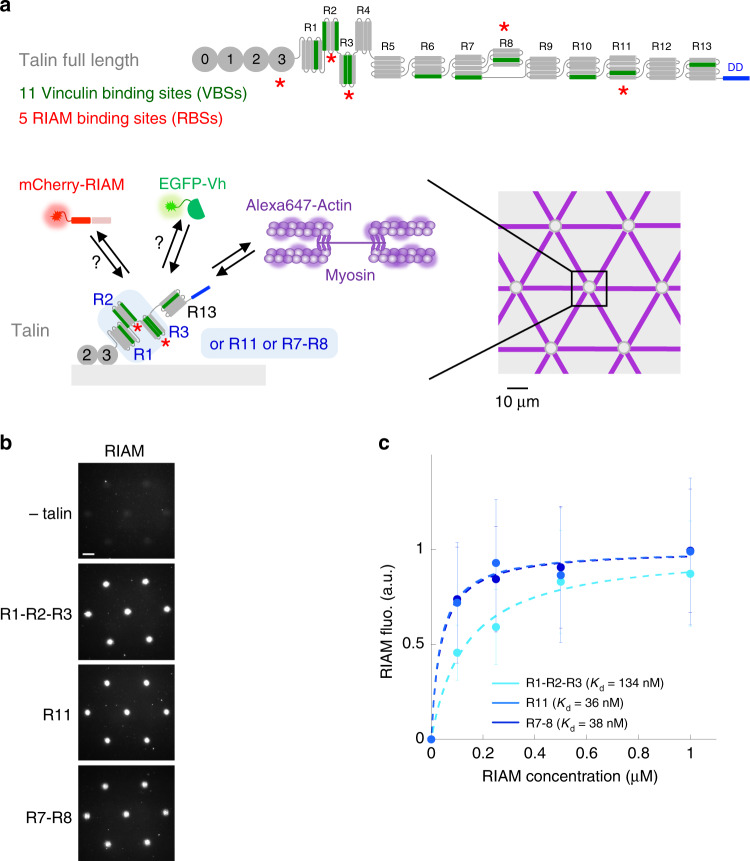
Fig. 1. In vitro reconstitution of talin–RIAM complexes.
a Top panel: organization of full-length talin featuring RIAM- and vinculin-binding sites. The vinculin-binding sites (VBSs) are the dark green helices. RIAM binds to the R2, R3, R8, and R11 domains of talin. R13 is the C-terminal actin-binding domain (ABD) of talin. Bottom panels: basic principle of the in vitro microscopy assay. Alexa647-labeled actin and myosin II self-assemble to apply force to talin R1–R2–R3, R11, or R7–R8 immobilized in micropatterns, which controls the binding of EGFP-vinculin head (EGFP-Vh) and mCherry-RIAM. Talin is represented as a monomer for convenience but it contains a dimerization domain (DD). b Representative images of the fluorescence of mCherry-RIAM 1-306 (1 µM) in non-coated control disks and disks coated with 1 µM talin R1–R2–R3 or R11, or R7–R8. Scale bar = 10 µm. This experiment was repeated three times independently with the same results. c Binding of RIAM to disks coated with talin R1–R2–R3, R11, and R7–R8. Conditions: 0–1 µM mCherry-RIAM 1-306, 1 µM of talin during the coating step. Data are mean ± SD. n = 150 disks all points, except (R1–R2–R3 + 0.5 µM RIAM) n = 137 disks. Source data are provided as a Source data file.