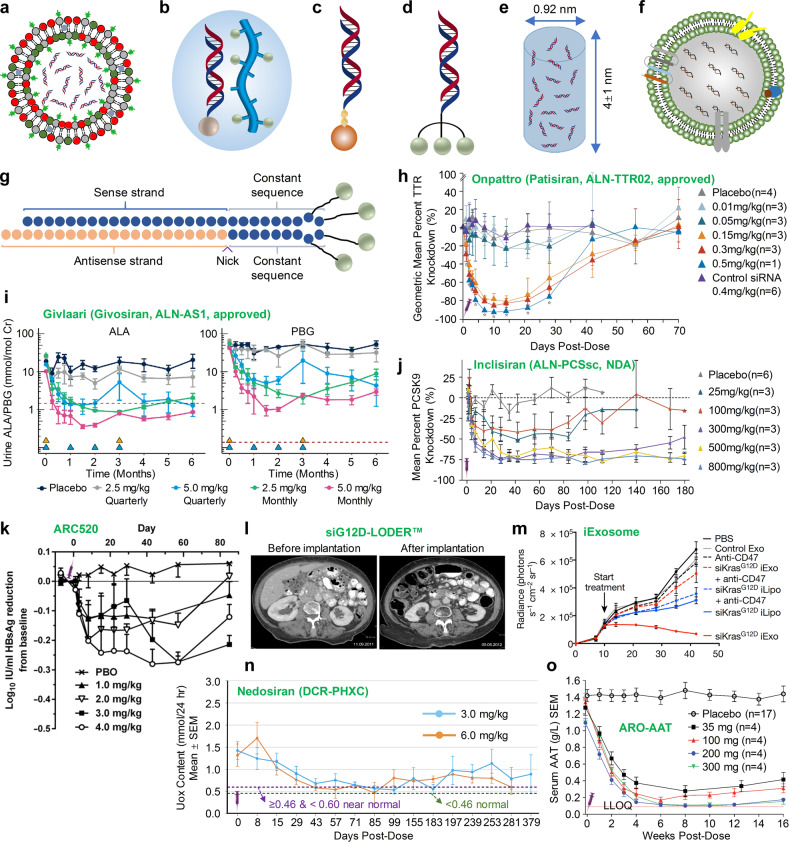
Fig. 5.
Schemes of representative clinically studied siRNA formulations and their pharmacodynamic performance. a–g Schemes of a lipid nanoparticles, b DPC™ or EX-1™, c TRiM™, d GalNAc–siRNA conjugates, e LODER™, f iExososme and g GalXC™. h Efficacy of ONPATTRO® (a liposome formulation) in healthy volunteers, in which siRNA targeting PCSK9 is formulated in lipid nanoparticles.34 Copyright Massachusetts Medical Society, 2013. i Levels of urinary aminolevulinic acid (ALA) and porphobilinogen (PBG) after once-monthly and once quarterly doses of GIVLAARI™ in acute hepatic porphyria (AHP) patients. Copyright Alnylam Pharmaceuticals, 2019. j Reduction of plasma PCSK9 after a single increasing dose of inclisiran.38 Copyright Massachusetts Medical Society, 2017. k Serum HBsAg reduction in hepatitis B patients who received the treatment of a single dose of ARC-520 (1–4 mg/kg) (formulated with DPC2.0) on a background of daily oral NUCs.264 PBO, patients on NUC therapy receiving placebo injection. NUCs nucleos(t)ide viral replication inhibitors. The error is shown as the SEM. Copyright American Association for the Advancement of Science, 2017. l Antitumor effect of siG12D-LODER™ combined with chemotherapy in locally advanced inoperable pancreatic cancer in a patient. A computed tomography (CT) scan was performed before and after (nine months later) the administration of siG12D-LODER™. The tumor measured 35.42 and 26.16 mm in the longest diameter, respectively. m PANC-1 tumor growth in animals receiving iExosomes or other control formulations.238N = 3 mice per group. Copyright Macmillan Publishers Limited, part of Springer Nature, 2017. n The 24-h Uox (urinary oxalate) values of healthy volunteers and patients treated with a single dose of Nedosiran (DCR-PHXC, a GalXC™ therapeutic) (3 and 6 mg/kg).265 Copyright Dicerna Pharmaceuticals, 2020. o Reduction of serum AAT in volunteers receiving a single dose of ARO-AAT (a TRiM™ therapeutic, 35–300 mg).266 The error bars show the SEM. Copyright Arrowhead Pharmaceuticals, 2019