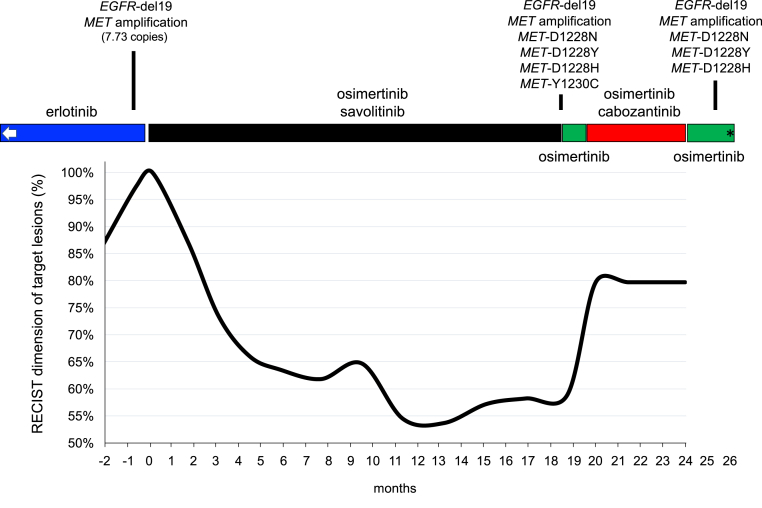
Figure 1.
The clinical, radiographic, and genomic course of EGFR-mutant NSCLC with MET-mediated resistance before, during, and after osimertinib plus savolitinib therapy. Graphical representation of RECIST target lesion measurements in percentage (baseline set at 100% at the time before the commencement of osimertinib plus savolitinib) over the course of therapy. Noted are different types of kinase inhibitors targeting EGFR (erlotinib and osimertinib) or targeting MET (savolitinib and cabozantinib). The results of tissue-based and liquid biopsy–based comprehensive genomic profiling results are highlighted in accordance with the course of therapy. The tissue-based comprehensive genomic profiling test (FoundationOne) at the time of erlotinib resistance also revealed additional genomic aberrations, including TP53 N239I, FGF14 amplification, and IRS2 amplification. The liquid biopsy–based (FoundationOne Liquid) tests after osimertinib and savolitinib also identified the TP53 N239I mutation. EGFR-del19 indicates EGFR exon 19 deletion (delL747_T751). The left white arrow indicates previous periods of erlotinib use; ∗ indicates the time of death. A note is made that this occurred during the COVID-19 pandemic in Massachusetts and no autopsy or SARS-CoV-2 testing was performed. COVID-19, coronavirus disease 2019; RECIST, Response Evaluation Criteria in Solid Tumor; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.