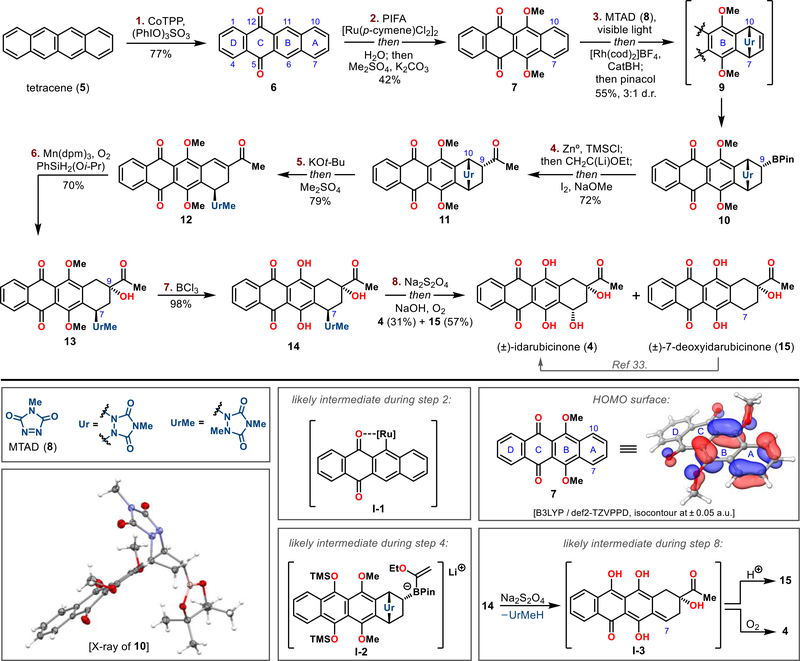
Figure 3.
Synthesis of (±)-idarubicinone (4) from tetracene (5). Reagents and conditions: 1. CoTPP (5 mol %), (PhIO)3SO3, CH2Cl2, 25 °C, 2 h, 77%; 2. [Ru(p-cymene)Cl2]2 (2.5 mol %), PIFA, DCE, 100 °C, 12 h; then H2O, 100 °C, 12 h; then Me2SO4, K2CO3, (CH3)2CO, 74 °C, 24 h, 42%; 3. MTAD (8), CH2Cl2, −50 °C, 36 h; then [Rh(cod)2]BF4 (10 mol %), dppb (10 mol %), HBcat, THF, −30 °C, 12 h; then pinacol, −78 to 25 °C, 12 h, 55% (3:1 dr); 4. Zn0, TMSCl, THF, ultrasonication, 40 °C, 30 min; then then CH2C(Li)OEt, −78 to −25 °C, 30 min; then I2, −78 to −25 °C, 30 min; then NaOMe, −78 to 25 °C, 4 h; then 1 M HCl, 25 °C, 2 h, 72%; 5. KOt-Bu, THF, −78 °C, 20 min; then Me2SO4, −78 to 0 °C, 1.5 h, 79%; 6. Mn(dpm)3 (10 mol %), PhSiH2(Oi-Pr), O2, i-PrOH/DCE 1:1, 0 to 25 °C, 2 h, 70%; 7. BCl3, CH2Cl2, −78 °C, 1 h, 98%; 8. Na2S2O4, H2O/THF/MeOH 1:1:1, −20 °C, 20 min; then NaOH, −20 °C, 60 s; then O2, −20 °C, 5 min, 31% of 4 and 57% of 15.