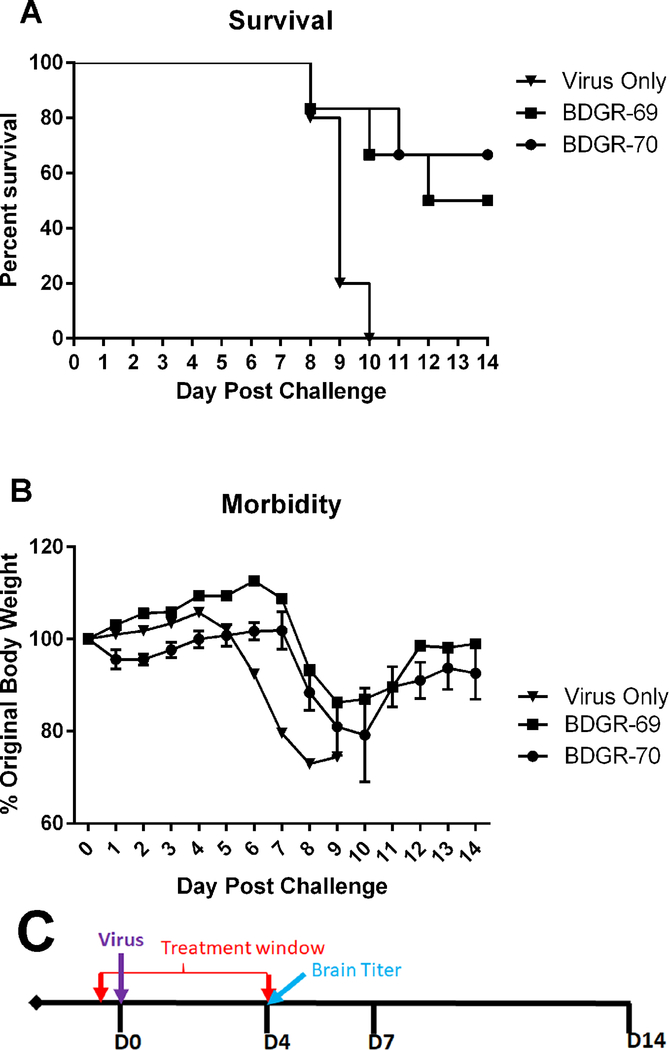
Figure 3. Prophylactic efficacy of BDGR-69 and -70 tested in C3H/HeN mice and challenged intranasally with VEEV TC-83.
Groups of six C3H/HeN mice per treatment, vehicle, BDGR-69 or BDGR-70 at 50 mg/kg/day, were assessed for (A) survival and (B) morbidity over 14 days following a s.c. challenge with VEEV TC-83. (C) Treatments were administered i.p. in a volume of 0.1 ml in 21.4% PEG400/8.6% RH40/70% water starting at 2 hours prior to virus infection (D0) and were given BID for 5 days (D0-D4).