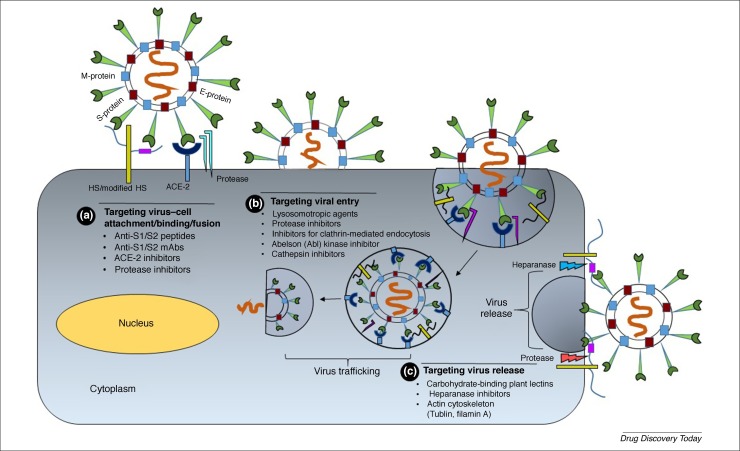
Figure 1.
Developing candidates that interfere with the early stages of virus-receptor attachment and internalization. (a) The early severe acute respiratory syndrome coronavirus (SARS-CoV-2) spike protein–host cell surface heparan sulfate proteoglycan (HSPG) interaction could be targeted by peptide antagonists that mimic S1/S2 subunits, or ACE2 sequence, or are heparin sulfate (HS) mimetics, or anti-Spike glycoprotein (S) antibodies. Likewise, protease inhibitors could prevent fusion with the angiotensin-converting enzyme 2 (ACE2) receptor. (b) The next step of SARS-CoV-2 invasion involving S binding to its fusion receptor (ACE2) offers the possibilities of using soluble ACE2 peptide or anti-ACE2 antibodies. Likewise, inhibitors of endocytosis or cathepsin L could reduce the efficiency of virus–cell fusion. The release of virus from the host cell could also be targeted through inhibitors of proteases or heparanase, which contribute to the process. For example, HS mimetics combined with a cocktail of protease inhibitors might block virus egress. Not shown is the role of other enzymes involved in viral replication, including helicase and RNA-dependent RNA polymerase, which offer a major route to small-molecule discovery. Abbreviation: mAb, monoclonal antibody.