Abstract
Background:
Palliative care consultation (PCC) is recommended for older adults hospitalized with cardiopulmonary conditions, but frequently is reserved for patients with malignant conditions and those with advanced age.
Objectives:
To compare age-adjusted PCC trends and the relationship between increasing age and PCC among older adults with cardiopulmonary and malignant conditions.
Methods:
Observational analysis of patients age ≥65 years, stratified by age and cardiopulmonary (heart failure, chronic obstructive pulmonary disease) vs. malignant (lung and gastrointestinal) conditions. Age-adjusted PCC trends over time and compound annual growth rates (CAGR) were compared.
Results:
Discharges with cardiopulmonary vs. malignant conditions were older, more likely to be female, and white. Relative to malignant conditions, discharges with cardiopulmonary conditions had lower age-adjusted PCC rates but higher CAGRS. Increasing age was associated with PCC in both groups but had a stronger effect among cardiopulmonary conditions.
Conclusions:
Older adults with cardiopulmonary conditions experienced lower rates of PCC, but higher rates of growth over time relative to those with malignant conditions.
Keywords: Palliative care, chronic obstructive pulmonary disease, heart failure, cancer, dementia, hospice
INTRODUCTION
Older adults in the United States (U.S.) are living longer with multiple chronic conditions1 and more functional deficits2, with increasingly complex and potentially unmet palliative care needs as a population. Older adults report limited social supports and burdensome symptoms, both of which contribute to hospitalizations and progressive disability.2, 3 Furthermore, this population frequently experience poor hospitalization and post-hospitalization outcomes including utilization of intensive care units near the end of life and high rates of post-discharge mortality. Access to palliative care services may play an important role in improving these outcomes, especially in light of the more than 40% of older adults who have not discussed goals of care with family or healthcare providers.4
Over the past decade there has been a substantial increase in the availability of palliative care services in the U.S. Ninety-four percent of hospitals with 300 or more beds offer palliative care consultation (PCC) teams.5 In parallel, the integration of palliative care into the care of older adults with multiple chronic conditions has garnered increasing interest, becoming practice and research priorities. Despite this momentum, a majority of older adults hospitalized with heart failure and chronic obstructive pulmonary disease (COPD) do not receive palliative care.6 At least 7% and 10% of older adults have heart failure or COPD in the U.S. respectively, with both conditions contributing to high rates of morbidity, mortality, and impaired quality of life.7–9 Moreover, these patients suffer from significant symptoms and symptom burden, the number and severity of which are similar to those reported by patients with cancer.10 Despite the need for palliative care among patients with heart failure and COPD, PCC is often reserved for adults with advanced age and those with malignant conditions. In a study estimating the point prevalence of PCC at 33 U.S. hospitals, 29% of adults ages 65 – 85 who were deemed appropriate for referral to palliative care received a PCC, compared to 40% of those 85 years of age and older.6 PCC rates also varied widely with underlying condition, with 35.8% of patients with cancer receiving a PCC compared with 26.1% with COPD and 21.6% for patients with heart failure.6
While many older adults have unmet palliative care needs regardless of age, it remains unknown if the growth of palliative care programs in U.S. hospitals has translated into increased PCC for older adults with heart failure and COPD over time, or if age- and condition-related differences in PCC persist. Therefore, to fill this knowledge gap, we compared PCC trends and growth rates among older adults with cardiopulmonary and malignant conditions by age during hospitalization to identify differences in PCC rates across conditions and age strata. We then evaluated the relationship between age strata and other demographic, clinical, and hospital characteristics and PCC for older adults by condition group.
METHODS
Study Design
This study was an observational study of data from the Healthcare Cost and Utilization Project’s National Inpatient Sample (NIS) of adults 65 years of age and older hospitalized between calendar years 2008 and 2014. Developed by the Agency for Healthcare Research and Quality, the NIS is an approximate 20% stratified cluster sample of acute-care hospitalizations from 44 states and is the largest publicly-available all-payer healthcare database in the United States.11 Findings from this study are reported in accordance with the STROBE guidelines.12
Sample
The NIS contains de-identified patient information at the level of hospital discharges (alive and decedent). Each discharge contains up to 30 International Classification of Disease 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes, and 15 ICD-9-CM procedural codes. To facilitate the use of multiple diagnosis codes, AHRQ has developed Clinical Classification Software (CCS) codes, which collapses ICD-9-CM diagnosis codes into approximately 300 clinically meaningful CCS categories.13
To determine trends in PCC, we created cohorts of discharges comprised of heart failure and COPD and malignant conditions (gastrointestinal cancer, lung cancer) as a comparator. We selected malignant conditions common among adults of both sexes 65 years of age and older and that are associated with significant morbidity and mortality.14–16 We included hospital discharges for patients who were 65 years or older, and stratified into two non-malignant groups by primary diagnosis: heart failure (HF, ICD-9-CM 428.X, 398.91, 402.01, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4 – 425.9) and COPD (416.8, 416.9, 490.0 – 505.0, 506.4, 508.1, 508.8). For the malignancy-related diagnoses cohort, we included discharges with a primary diagnosis of gastrointestinal cancer (CCS 12–18) or lung cancer (CCS 19 or 20).13 We excluded discharges with a major operation during the admission identified by ICD-9-CM code, those with a cancer diagnosis in any position other than principal diagnosis among the non-oncologic cohort, or those with other cancer diagnoses in any position other than primary in the oncologic cohort.
Primary Outcome
The primary outcome was PCC during the study period. We identified PCC by the presence of an ICD-9-CM code for “Encounter for Palliative Care” (V66.7). The ICD-9-CM defines this code as instances of “palliative care”, “end-of-life care”, “hospice care”, or “terminal care”.17
Variables of Interest and Covariates
We included demographic and clinical covariates based on prior research examining associations between health conditions and PCC.6, 18, 19 Demographic and clinical variables of interest included sex, age at discharge, race, Charlson comorbidity index (CCI), and discharge year. We stratified age into mutually exclusive increments of 65–69, 70–74, 75–79, 80–84 and ≥85 years of age. Race was classified according to HCUP race/ethnicity groupings as white, black, Hispanic, Asian and other.11 Because of the high rate of missing race data, particularly in the earlier years of the dataset, missing race was included as its own category in analyses. We used the CCI to adjust for the effect of comorbid disease in all study populations. The CCI was calculated by ICD-9-CM codes for a designated list of comorbid diseases and is available in the NIS.11 Because of the relative infrequency of no comorbidity in our cohort, discharges were classified according to the following CCI groups for modeling purposes: 0–1, 2, and 3 or more.
Hospital factors included hospital region, tax status (e.g. non-profit, for-profit), urbanicity, teaching status, and bedsize. Region was classified according to the four US census regions of Northeast, Midwest, South, and West. Hospitals were classified as urban or rural based upon their proximity to a metropolitan area and teaching or non-teaching based on the presence of residency training programs. Hospital bedsize was classified as small, medium, or large. HCUP determines hospital bedsize based on location-specific characteristics including teaching status, urbanicity, and region,11 which vary across facilities.
Regression models were also adjusted for discharge year, payer status, and admission status. Discharge year was included in regression models to adjust for annual changes in the frequency of PCC. Payer status was listed by the primary payer for the hospitalization, and given the geriatric population, classified according to the most prevalent groups as Medicare, privately insured, or other. Discharge admission status was categorized first as either an elective or an urgent/emergent admission. Secondly, they were classified according to whether they were admitted through the emergency department (ED), as opposed to directly admitted.
Statistical Analysis
All analyses used methods for complex surveys consistent with the stratified cluster design of the NIS. We first calculated age-adjusted rates in PCC by group using population estimates of non-malignant and malignant conditions from the NIS. We then calculated the compound annual growth rate (CAGR) for each age category for each condition to quantify temporal trends of PCC growth. The CAGR is a measure of growth over multiple time periods, representing the geometric average rate of growth from year to year. The CAGR is frequently used in health services research as an evaluative measure of healthcare trends.20 We compared age-adjusted rates of PCC by condition using one-way Analysis of Variance. We tested for statistical significance in trends over time and for differences in CAGRs by age group for each condition using univariable students’ t-tests and linear regression, respectively.
We compared study variables using the Rao-Scott chi-square test for categorical variables. To evaluate factors associated with PCC, we performed separate hierarchical multivariable logistic regression for each group (heart failure, COPD, cancer), adjusting for previously mentioned covariates. All statistical tests were two-sided, and statistical significance was set at p-value < 0.01. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). This study was determined to be non-human subjects research, and therefore formal review was not required by the Yale Institutional Review Board.
RESULTS
Descriptive Statistics
The total sample was comprised of 7,072,079 (heart failure=4,105,465 (58%); COPD=2,966,614, (42%) and 608,087 malignancy-related patient discharges. Discharges with non-malignant compared to malignant conditions were more likely to be female (57.5% vs. 48.1%), older (age ≥85; 26.5% vs. 17.2%), and white (68.8% vs. 66.1%). (Table 1.; p<.001 for all) When examined by individual conditions, discharges with heart failure accounted for the differences in age observed, with 33.7% of patients age ≥85 compared with COPD = 16.4% and 17.2% of malignancy-related discharges. Discharges with non-malignant conditions had a lower comorbidity burden, with 65% of heart failure and 34.6% of COPD discharges affected by ≥3 comorbidities, compared with 85.2% of the malignant group.
Table 1.
Sample demographics and clinical characteristics by non-malignant and malignant conditions
| Total Non-Malignant (n = 7,072,079) | Heart Failurec (n = 4,105,465) | Chronic Obstructive Pulmonary Diseasec (n = 2,966,614) | Malignantc (n = 608,087) | |
|---|---|---|---|---|
| Age in years, n (%) | ||||
| 65–69 | 1,269,981 (18) | 559,342 (13.6) | 710,639 (24.0) | 143,353 (23.6) |
| 70–74 | 1,290,573 (18.2) | 620,699 (15.2) | 669,874 (22.6) | 133,206 (21.9) |
| 75–79 | 1,311,291 (18.5) | 712,725 (17.4 | 598,566 (20.2) | 123,468 (20.3) |
| 80–84 | 1,329,994 (18.8) | 828,192 (20.2) | 501,802 (16.9) | 103,488 (17.0) |
| ≥85 | 1,870,238 (26.4) | 1,384,506 (33.7) | 485,732 (16.4) | 104,572 (17.2) |
| Sex = Female, n (%) | 4,067,450 (57.5) | 2,287,678 (55.7) | 1,779,772 (60.0) | 292,261 (48.1) |
| Race/Ethnicitya, n (%) | ||||
| White | 4,869,002 (68.8) | 2,765,971 (67.4) | 2,103,031 (70.9) | 402,142 (66.1) |
| Black | 752,885 (10.6) | 490,992 (12.0) | 261,893 (8.8) | 67,380 (11.1) |
| Hispanic | 432,337 (6.1) | 259,671 (63.2) | 172,666 (5.8) | 39,596 (6.5) |
| Asian | 116,935 (16.5) | 70,419 (17.1) | 46,516 (15.7) | 20,121 (3.3) |
| Other | 181,054 (2.6) | 106,291 (25.9) | 74,763 (2.5) | 17,796 (2.9) |
| Charlson Comorbidity Index, n (%) | ||||
| CCI ≤1 | 1,576,504 (22.3) | 522,333 (12.7) | 1,054,171 (35.5) | 2,049 (0.3) |
| CCI 2 | 1,798,455 (25.4) | 913,224 (22.2) | 885,231 (29.8) | 87,970 (14.5) |
| CCI ≥3 | 3,697,119 (52.3) | 2,669,908 (65.0) | 1,027,211 (34.6) | 518,068 (85.2) |
| Hospital Region, n (%) | ||||
| Northeast | 1,482,809 (21.0) | 883,390 (21.5) | 599,419 (20.2) | 132,527 (21.8) |
| Midwest | 1,678,572 (23.7) | 976,123 (23.8) | 702,449 (23.7) | 138,860 (22.8) |
| South | 2,880,074 (40.7) | 1,628,123 (39.7) | 1,251,951 (42.4) | 232,618 (38.3) |
| West | 1,030,623 (14.6) | 617,829 (15.0) | 412,794 (13.9) | 104,082 (17.1) |
| Tax statusb, n (%) | ||||
| Government | 831,592 (11.8) | 467,867 (11.4) | 363,725 (12.3) | 72,754 (12.0) |
| Not-for-Profit | 5,101,853 (72.1) | 3,019,699 (73.5) | 2,082,154 (70.2) | 454,204 (74.7) |
| For-Profit | 1,106,361 (15.6) | 599,556 (14.6) | 506,805 (17.1) | 78,093 (12.8) |
| Urban hospitalb, n (%) | 5,690,930 (80.5) | 3,390,930 (82.6) | 2,300,000 (77.5) | 533,553 (87.7) |
| Teaching hospital, n (%) | 2,628,795 (37.2) | 1,645,450 (40.1) | 983,345 (33.1) | 290,699 (74.8) |
| Hospital bedsizeb, n (%) | ||||
| Small | 1,256,067 (17.8) | 692,178 (16.9) | 563,889 (19.0) | 79,185 (13.0) |
| Medium | 1,871,454 (26.5) | 1,072,870 (26.1) | 798,584 (26.9) | 150,002 (24.7) |
| Large | 3,912,285 (55.3) | 2,322,074 (56.6) | 1,590,211 (53.6) | 375,863 (61.8) |
| Palliative care, n (%) | 150,137 (2.1) | 113,688 (2.8) | 36,449 (1.2) | 105,637 (17.4) |
| Died during hospitalization, n (%) | 197,343 (28.0) | 150,953 (3.7) | 46,390 (1.6) | 608,087 (13.4) |
Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; CCI, Charlson Comorbidity Index
Missing 10% of the sample;
Missing ≤0.5% of the sample; column totals may not equal 100% due to rounding;
p-values <0.001 for all associations among health conditions and demographic and clinical characteristics
Temporal Trends
Age-adjusted rates of PCC were lower for discharges with heart failure and COPD overall and in each year compared to patients with malignant conditions (Figure 1; p <.001). Among the total number of discharges with heart failure, PCC increased from 10.1 per 1,000 discharges in 2008 to 43.9 per 1,000 in 2014 (p <.001). Among the total number of discharges with COPD, PCC rates increased from 6.8 per 1,000 discharges to 18.1 per 1,000 in 2014 (p <.001). Among the total number of discharges with malignant conditions, PCC also increased from 106 per 1,000 discharges in 2008 to 233 per 1,000 discharges in 2014 (p <.001). For each condition, age-adjusted rates of PCC were highest among discharges 85 years of age and remained the highest across all study years (p <.001). Based on the trend data for each condition, CAGRS were highest among discharges with heart failure (total sample = 23.4%), followed by COPD (total sample = 15.0%), and malignant conditions (total sample = 11.9%) across age strata (p<.001). An increasing trend in CAGRS by age group was observed among discharges with malignant conditions (β = .001; p = 0.04), but not heart failure (β = −.000; p = 0.2) or COPD (β = −.001; p = 0.1) (Figure 1).
Figure 1.
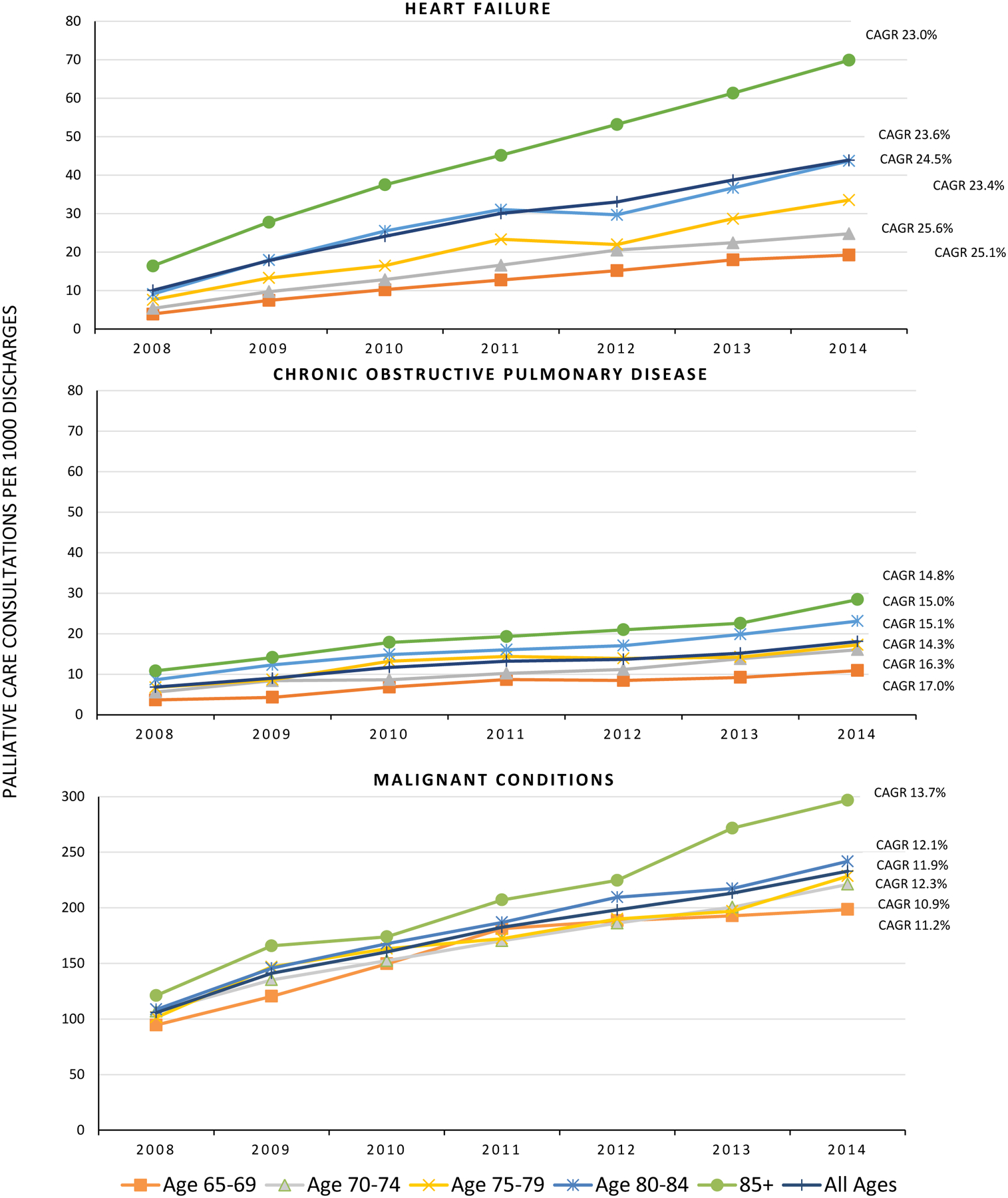
Age-adjusted palliative care consultations per 1,000 older adults with heart failure, COPD, and malignancy
P<.001 for comparison of PCC age-adjusted rates by year by condition (heart failure, COPD, malignancy); P<.001 for trend for all age strata for all conditions; P = 0.2 for association between CAGRS and age group for heart failure; P = 0.1 for association between CAGRS and age group for COPD; P = 0.04 for association between CAGRS and age group; CAGRS = Compound Annual Growth Rates
Multivariable Regression Analysis of Palliative Care Consultation
Non-malignant conditions
Increasing odds of PCC by age stratum were observed across conditions but were most pronounced among discharges with heart failure. Discharges who age 85 years with heart failure and COPD were more than twice as likely to receive a PCC during hospitalization compared to discharges aged 65 – 69 (heart failure Odds Ratio (OR) 3.94, 95% CI 3.70 – 4.19; COPD OR 2.61, 95% CI 2.40 – 2.83) (Table 2). Discharges with heart failure and COPD who were black (heart failure OR 0.62, 95% CI 0.58 – 0.66; COPD OR 0.52, 95% CI 0.47 – 0.58) or Hispanic (heart failure OR 0.72, 95% CI 0.66 – 0.79; COPD 0.53, 95% CI 0.45 – 0.62) were less likely to receive a PCC compared with whites.
Table 2.
Adjusted multivariable logistic regression of palliative care consultation by conditiona
| Heart Failure (OR, 95% CI) | Chronic Obstructive Pulmonary Disease (OR, 95% CI) | Malignant (OR, 95% CI) | |
|---|---|---|---|
| Age (years) | |||
| 65 – 69 | -- | -- | -- |
| 70 – 74 | 1.30 (1.21 – 1.40) | 1.41 (1.30 – 1.53) | 1.14 (1.09 – 1.02) |
| 75 – 79 | 1.70 (1.59 – 1.83) | 1.70 (1.57 – 1.85) | 1.23 (1.17 – 1.29) |
| 80 – 84 | 2.32 (2.18 – 2.48) | 2.15 (1.98 – 2.34) | 1.39 (1.31 – 1.46) |
| ≥85 | 3.94 (3.70 – 4.19) | 2.61 (2.40 – 2.83) | 1.74 (1.65 – 1.84) |
| Female sex (referent = male) | 0.99 (0.96 – 1.02) | 0.96 (0.91 – 1.01) | 1.09 (1.05 – 1.12) |
| Race/Ethnicity | |||
| White | -- | -- | -- |
| Black | 0.63 (0.59 – 0.67) | 0.52 (0.47 – 0.58) | 0.92 (0.85 – 0.99) |
| Hispanic | 0.72 (0.66 – 0.79) | 0.53 (0.45 – 0.62) | 0.78 (0.68 – 0.88) |
| Asian | 0.75 (0.66 – 0.86) | 0.62 (0.48 – 0.80) | 0.86 (0.76) – 0.98) |
| Other | 0.80 (0.70 – 0.91) | 0.64 (0.52 – 0.79) | 0.87 (0.74 – 1.02) |
| Charlson Comorbidity Index (CCI) | |||
| CCI ≤1 | -- | -- | |
| CCI 2 | 0.88 (0.83 −0.93) | 0.94 (0.88 – 1.00) | 1.78 (1.26 – 2.51) |
| CCI ≥3 | 1.20 (1.14 – 1.27) | 1.05 (0.98 – 1.11) | 2.38 (1.69 – 3.36) |
| Hospital Region | |||
| Northeast | -- | -- | -- |
| Midwest | 1.41 (1.28 – 1.55) | 1.41 (1.25 – 1.60) | 1.12 (1.00 – 1.26) |
| South | 1.17 (1.06 – 1.31) | 1.13 (0.99 – 1.29) | 1.27 (1.14 – 1.55) |
| West | 1.72 (1.55 – 1.91) | 2.01 (1.73 – 2.34) | 1.33 (1.14 – 1.55) |
| Tax Status | |||
| Not-for-profit | -- | -- | -- |
| Government | 0.78 (0.70 – 0.88) | 0.83 (0.73 – 0.95) | 0.82 (0.73 – 0.93) |
| For-profit | 0.59 (0.53 – 0.66) | 0.62 (0.51 – 0.75) | 0.61 (0.53 – 0.70) |
| Urban hospital (referent = rural) | 1.67 (1.50 – 1.86) | 1.82 (1.57 – 2.10) | 1.13 (1.00 – 1.27) |
| Teaching hospital (referent = non-teaching) | 1.28 (1.19 – 1.38) | 1.49 (1.32 – 1.67) | 1.01 (1.00 – 1.22) |
| Hospital bedsize | |||
| Small | -- | -- | -- |
| Medium | 1.32 (1.21 – 1.44) | 1.44 (1.26 – 1.64) | 1.09 (0.93 – 1.28) |
| Large | 1.50 (1.38 – 1.63) | 1.62 (1.44 – 1.82) | 1.09 (0.93 – 1.28) |
Also controlling for missing race, discharge year, payer, and elective vs. emergent admission
There were significant associations between hospital characteristics and PCC. Among both heart failure and COPD, discharges from urban, teaching, medium, or large bedsize hospitals were more likely to receive a PCC during hospitalization. Whereas discharges with heart failure or COPD hospitalized in a for-profit hospital were 59% and 62% less likely to receive a PCC (heart failure OR 0.59, 95% CI 0.53 – 0.66; COPD OR 0.62, 95% CI 0.51 – 0.75) during the study period.
Malignant conditions
For discharges with malignant conditions, those ≥85 years were more likely to receive a PCC during hospitalization compared to discharges age 65 – 69, though this difference was smaller in magnitude compared to patients with non-malignant conditions (OR 1.74 95% CI 1.65 – 1.84). Increasing comorbidity burden was associated with PCC (Referent = CCI 0–1; CCI ≥3; OR 2.38, 1.69 – 3.36). In contrast to the non-malignant group, hospitalization characteristics (urbanicity, teaching status, and bedsize) were not associated with PCC among patients in the malignant group. However, similar to discharges with heart failure and COPD, discharges from for-profit hospitals were associated with 49% decreased likelihood of receiving PCC (OR 0.61 (0.53 – 0.70).
DISCUSSION
In a nationally representative age-adjusted sample of hospital discharges aged 65 years of age and older with heart failure, COPD, and malignant conditions, we found increasing trends in rates of PCC. Discharges with heart failure and COPD had significantly lower age-adjusted rates of PCC compared to discharges with malignant conditions overall. However, discharges with heart failure and COPD did have higher growth rates of PCC utilization compared to discharges with malignant conditions, although the absolute rates of PCC remain dwarfed compared with malignant conditions. Age continued to be one of the most consistent factors associated with PCC, with discharges 85 years having the highest age-adjusted rates of PCC overall across conditions and study years.
These findings align with Szekendi and colleagues6 who found that among patients appropriate for palliative care, 29% of those 65–84 years of age received a PCC compared with 40% of adults 85 years. Together, these findings may reflect increasing risk of mortality, particularly during hospitalization, as age increases. Others have found that providers may act as gatekeepers to palliative care, and may be influenced by their own perceptions of the usefulness and purpose of palliative care, as well as their perceptions of the need and appropriateness of the patient for referral.21, 22 This may be particularly true for patients with heart failure and COPD, for whom prognosis is less predictable compared with cancer-related conditions.23 In this context, our findings may also reflect providers’ preferences for PCC referral, specifically for patients with advanced age, for whom aggressive measures particularly at the end of life are viewed to have limited benefit.
The magnitude of the age-adjusted PCC rates was significantly lower for patients with heart failure and COPD. However, we were encouraged to find that growth, measured using CAGRs, was 25–200% higher compared to discharges with malignant conditions. Recent heart failure and COPD guidelines recommend referral to PCC for patients with advanced disease.24, 25 In parallel, the past decade has seen increasing growth in the body of evidence emphasizing the value of palliative care for patients with heart failure and COPD.26, 27 Thus, the increased growth rates found in this study may represent increased recognition by clinicians of the benefit of PCC in non-malignant conditions like heart failure and COPD.
Palliative care availability and its workforce has expanded nationally, with growth of palliative care delivery systems centered in urban and academic medical centers. These changes could also affect the PCC CAGRs among heart failure and COPD discharges seen in the current study.9 We also found that hospital characteristics were associated with PCC. This aligns with past reports which found that penetration of palliative care has improved in large academic medical centers for patients with non-malignant, as well as malignant, conditions.5 Taken together, findings emphasize that efforts to increase PCC access for patients in small, rural, and for-profit hospitals are still necessary to enhance uniform delivery of high-quality, patient-centered care regardless of the healthcare facility.
CAGRs also likely reflect an increased demand for palliative services among older adults with serious life-limiting illnesses. This group will only increase in size as the population ages; however, specialist palliative care providers able to meet this need are projected to be in short supply.9,33–35 Currently, only 25% of PCC teams have clinician staffing that meets the Joint Commission’s standards for palliative care programs.28 Further, only 72% of hospitals with 50 beds or more have PCC services.9 PCC workforce shortages of sub-specialty clinicians trained in palliative care delivery remain a significant challenge.29 Adequate palliative care workforce planning, subspecialist and hospitalist training, and novel PCC delivery paradigms, including telehealth palliative care30, and primary palliative care31, will be critical to support the growing PCC needs for these highly complex and multimorbid older adults.
This study has several limitations. We measured PCC using the ICD-9-CM code for palliative care in the NIS data. Past reports indicate that specificity of the code is generally high32, 33, but sensitivity may vary, which could result in the underestimation of PCC. Our use of the ICD-9-CM code also likely captures palliative care delivered by palliative care specialists but not provided by other clinicians during hospitalization. In addition to the potential for misclassification of PCC, there is the potential for misclassification by health condition. However, we mitigated the likelihood of this limitation by using previously validated ICD-9 codes for the health conditions included in the analyses. We were also limited by our inability to classify the analysis by disease severity which may explain some of the variation in rates of PCC by age group and warrants further inquiry. However, while trends in PCC by age may be associated with disease severity, there is growing interest in involving palliative care earlier in the disease course.9, 34 Thus, our findings may represent a growing recognition of the value of palliative care for patients who are younger and with perhaps with less severe disease. Unlike other studies,35, 36 we did not limit our sample to discharges who died while hospitalized, and thus our sample may have included discharges who legitimately may not have had unmet palliative care needs. However, limiting the sample to hospital decedents would represent a narrow view of trends in PCC for decedents without accounting for the high burden of unmet palliative needs for patients throughout their disease trajectory. Trends relying on samples drawn from hospital decedents may also be more indicative of inpatient hospice use than palliative care support, which similarly would discount the importance of palliative care support for any patient with a life-limiting illness regardless of prognosis.37
Conclusions
Among a nationally representative sample of older adults, rates of age-adjusted PCC were highest among those >=85 years across health conditions. PCC rates were several fold lower for patients with heart failure and COPD compared to patients with malignant conditions across age strata, although growth rates for these conditions were higher. Given increased growth rates among patients with HF and COPD, findings suggest an increasing recognition of the value of PCC for these patients over time. Future studies should examine strategies and models for implementing palliative care for common and serious cardiopulmonary conditions.
Highlights.
In this observational study of adults 65 years of age and older with cardiopulmonary conditions and cancer, rates of age-adjusted palliative care consultation was highest among discharges with malignant conditions.
Compound annual growth rates of palliative care consultation were highest among discharges with cardiopulmonary conditions.
Older adults with cardiopulmonary conditions had lower age-adjusted rates of palliative care consultation, but higher growth over time.
ABBREVIATIONS LIST
- U.S.
United States
- PCC
Palliative Care Consultation
- COPD
Chronic Obstructive Pulmonary Disease
- NIS
National Inpatient Sample
- ICD-9-CM
International Classification of Diseases 9th Revision, Clinical Modification
- CCS
Clinical Classification Software
- CCI
Charlson Comorbidity Index
- HCUP
Healthcare Cost Utilization Project
- ED
Emergency Department
- CAGR
Compound Annual Growth Rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
This publication was supported by the Department of Veterans Affairs Office of Academic Affiliations through the National Clinical Scholars Program and the Pain Research, Informatics, Medical comorbidities and Education Center CIN 13–407. This publication was made possible by CTSA Grant Number TL1 TR001864 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
REFERENCES
- 1.Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: A systematic review and meta-analysis. Arch Gerontol Geriatr. 2016;67:130–138. doi: 10.1016/j.archger.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Patel KV, Guralnik JM, Phelan EA, et al. Symptom burden among community-dwelling older adults in the United States. J Am Geriatr So. 2019; 67(2):223–231. doi: 10.1111/jgs.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valtorta NK, Moore DC, Barron L, Stow D, Hanratty B. Older adults ‘ social relationships and health care utilization: a systematic review. 2018;108(4):1–10. doi: 10.2105/AJPH.2017.304256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kale MS, Ornstein KA, Smith CB, et al. End-of-life discussions with older adults. J Am Geriatr Soc. 2017;64(10):1962–1967. doi: 10.1111/jgs.14285.End-of-Life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center to Advance Palliative Care. America’s care of serious illness 2019 state-by-state report card on access to palliative care in our nation’s hospitals. https://reportcard.capc.org/. Accessed October 1, 2019. [DOI] [PMC free article] [PubMed]
- 6.Szekendi MK, Vaughn J, Lal A, Ouchi K, Williams MV. The prevalence of inpatients at 33 U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med. 2016;19(4):360–372. doi: 10.1089/jpm.2015.0236. [DOI] [PubMed] [Google Scholar]
- 7.Akgün KM, Crothers K, Pisani M. Epidemiology and management of common pulmonary diseases in older persons. J Gerontol A Biol Sci Med Sci. 2012;67(3):276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin. 2007;3(4):381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavalieratos D, Gelfman LP, Tycon LE, et al. Palliative care in heart failure: rationale, evidence, and future priorities. J American College of Card. 2017;70:1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekelman DB, Rumsfeld JS, Havranek EP, et al. Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J General Internal Med. 2009;24:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp Accessed January 11, 2019. [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 13.HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006–2009. Agency for Healthcare Research and Quality, Rockville, MD. www.hcupus.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed January 11, 2019. [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 15.Kochanek KD, Murphy SL, Xu J, Arias E, Ph D. Mortality in the United States. NCHS Data Brief. 2017; (293):1–8. [PubMed] [Google Scholar]
- 16.Rizzuto D, Melis RJF, Angleman S, Qiu C, Marengoni A. Effect of chronic diseases and multimorbidity on survival and functioning in elderly adults. J Am Geriatr Soc. 2017;65(5):1056–1060. doi: 10.1111/jgs.14868. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control. ICD - ICD-9-CM - Addenda, Conversion Tables, and Guidelines. 2009. http://www.cdc.gov/nchs/icd/icd9cm_addenda_guidelines.htm. Accessed January 27, 2019.
- 18.Penrod JD, Garrido MM, McKendrick K, et al. Characteristics of hospitalized cancer patients referred for inpatient palliative care consultation. J Palliat Med. 2017;20:1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beernaert K, Cohen J, Deliens L, et al. Referral to palliative care in COPD and other chronic diseases: a population-based study. Respiratory medicine. 2013;107:1731–1739. [DOI] [PubMed] [Google Scholar]
- 20.Palisch C, Dorsey ER. The anatomy of medical research. JAMA. 2015;22959(2):174–189. doi: 10.1001/jama.2014.15939. [DOI] [PubMed] [Google Scholar]
- 21.Ziehm J, Farin E, Seibel K, Becker G, Köberich S. Health care professionals’ attitudes regarding palliative care for patients with chronic heart failure: an interview study. BMC Palliative Care. 2016;15(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siouta N, Clement P, Aertgeerts B, Van Beek K, Menten J. Professionals’ perceptions and current practices of integrated palliative care in chronic heart failure and chronic obstructive pulmonary disease: a qualitative study in Belgium. BMC Palliative Care. 2018;17:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ. 2005;330(7498):1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanken PN, Terry PB, DeLisser HM, et al. An official American Thoracic Society clinical policy statement: Palliative care for patients with respiratory diseases and critical illnesses. American Journal of Respiratory and Critical Care Medicine. 2008;177:912–927. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–319. [DOI] [PubMed] [Google Scholar]
- 26.Morrison RS, Aldridge MD, Block J, Chiu L, Maroney C, Morrison CA, Meier DE. The National Palliative Care Research Center: ten years of promoting and developing research in palliative care. J Palliat Med. 2018;21:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie K, Gelfman L, Horton JR, Goldstein NE. State of research on palliative care in heart failure as evidenced by published literature, conference proceedings, and NIH Funding. Journal of Cardiac Failure. 2017;23(2):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spetz J, Dudley N, Trupin L, Rogers M, Meier DE, Dumanovsky T. Few hospital palliative care programs meet national staffing recommendations. Health Aff. 2016;35(9):1690–1697. doi: 10.1377/hlthaff.2016.0113. [DOI] [PubMed] [Google Scholar]
- 29.Kamal AH, Maguire JM, Meier DE. Evolving the palliative care workforce to provide responsive, serious illness care evolving the palliative care workforce. Ann Intern Med. 2015;163(8):637–638. doi: 10.7326/M15-0071. [DOI] [PubMed] [Google Scholar]
- 30.Durie C, Tanksley-Bowe C. Rural readmissions in the palliative care vacuum. J Hosp Palliat Nurs. 2018;20(2). [DOI] [PubMed] [Google Scholar]
- 31.Quill TE, Abernethy AP. Generalist plus specialist palliative care - creating a more sustainable model. N Engl J Med. 2013;368(13):1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 32.Hua M, Li G, Clancy C, Morrison RS, Wunsch H. Validation of the V66.7 Code for Palliative Care Consultation in a Single Academic Medical Center. Journal of palliative medicine. 2016;XX:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua M, Li G, Clancy C, Morrison RS, Wunsch H. Validation of the V66.7 Code for palliative care consultation in a single academic medical center. J Palliat Med. 2016;XX(Xx):1–6. doi: 10.1089/jpm.2016.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinke LF, Meier DE. Research priorities in subspecialty palliative care: policy initiatives. J Palliat Med. 2017;20(8):813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen JJ, Ko E, Kim P, et al. Life-Sustaining procedures, palliative care consultation, and do-not resuscitate status in dying patients with COPD in US hospitals: 2010–2014. J Pallit Care. 2018;33:159–166. [DOI] [PubMed] [Google Scholar]
- 36.Roeland EJ, Triplett DP, Matsuno RK, et al. Patterns of palliative care consultation among elderly patients with cancer. Journal of the National Comprehensive Cancer Network 2016;14 439–445. [DOI] [PubMed] [Google Scholar]
- 37.Ferrell BR, Twaddle AM, Meier DE. National consensus project clinical practice guidelines for quality palliative care. Journal of Palliative Medicine. 2018;21(12). 10.1089/jpm.2018.0431. [DOI] [PubMed] [Google Scholar]


