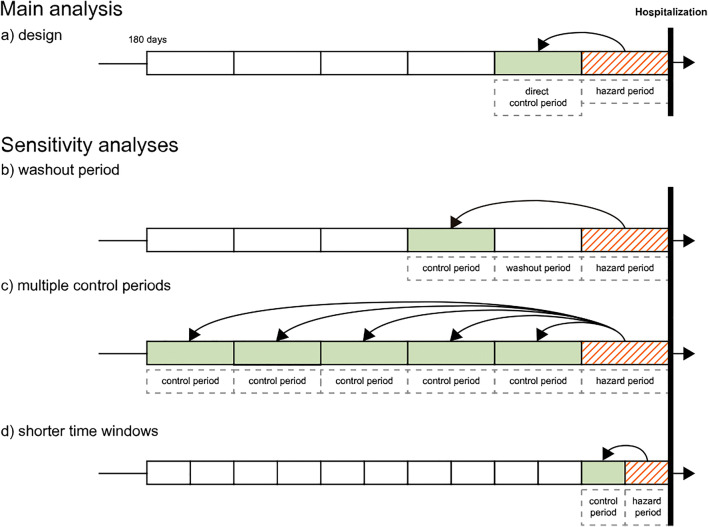
Fig. 1.
Case-crossover design. As a case-only design, patients serve both as cases and their own controls. Main analysis: For each patient, exposure in the “hazard” period immediately preceding the outcome was compared with exposure in the “direct control” period earlier in time (a). Hazard and control period were equal in length. Sensitivity analyses: Introduction of a single washout period to prevent exposure overlapping from the control into the subsequent hazard period (b). Inclusion of multiple control periods simultaneously to assess the impact of more distant periods and to account for persistent drug use (c). Finally, we evaluated shorter time periods for hazard and control periods (d)