Figure 3. Reversibility of Dr-FGFR1 and Dr-EGFR activation.
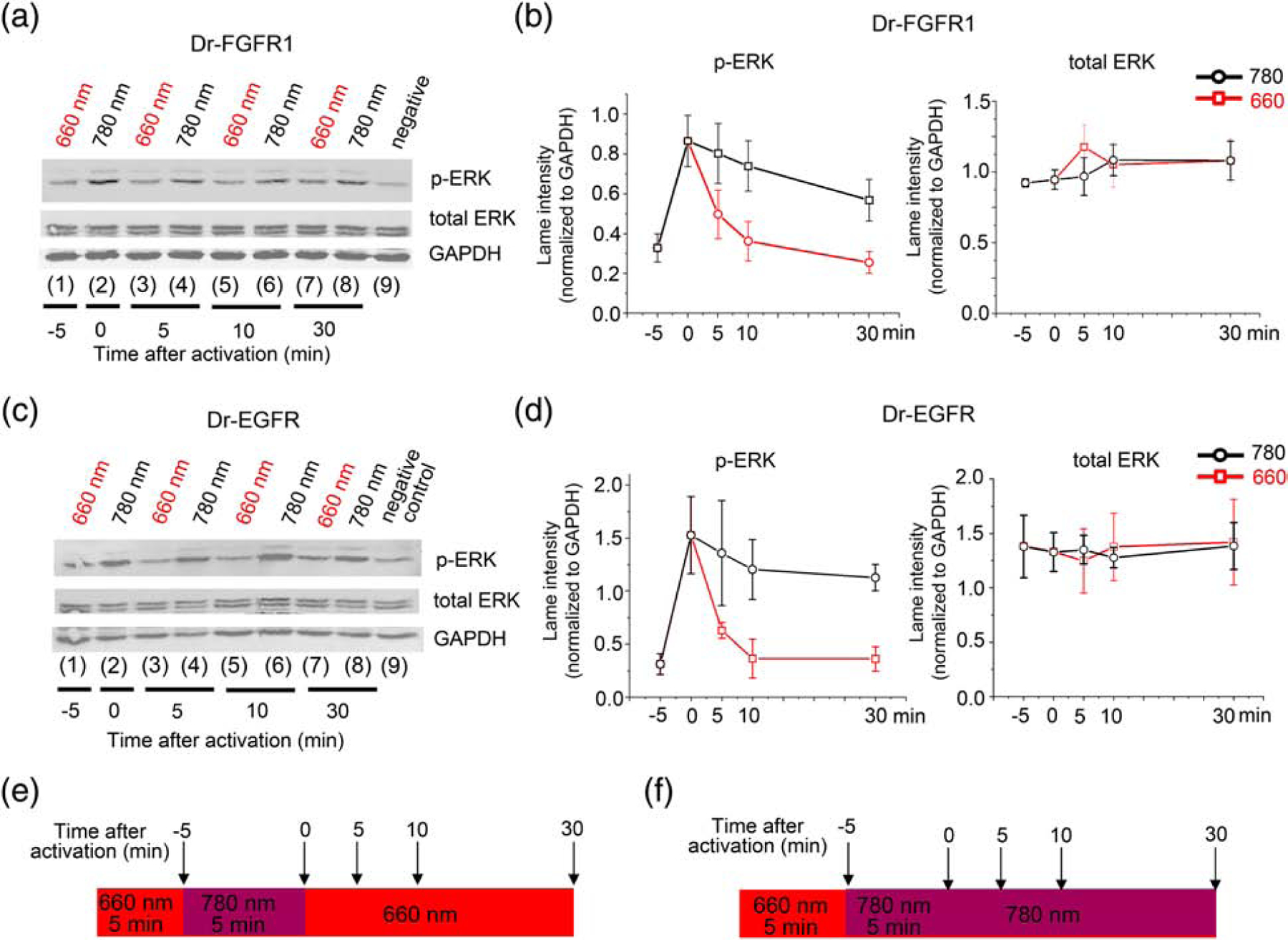
(a) Western blot of phospho-ERK1/2, total ERK1/2 and GAPDH in HeLa cell lysate. From left to right: cells co-transfected with Dr-FGFR1 and carrier DNA (1) kept under 660 nm light before induction; (2) induced for 5 min with 780 nm light; (3), (5) and (7) inactivated with 660 nm light for 5, 10 and 30 min, respectively, after 5 min of 780 nm induction. (4), (6) and (7) are controls of inactivation: cells were kept under 780 nm light for additional 5, 10 and 30 min after step (2). (9) are cells without Dr-FGFR1 as a negative control. (b) Quantification of lane intensities of phospho-ERK and total ERK, normalized to GAPDH represented as graphs. Left: downregulation of phospho-ERK1/2 upon action of 660 nm light (red line) and phospho-ERK1/2 downregulation in constantly activated with 780 nm light cells (black line). Right: total ERK1/2 levels. (c) Western blot of phospho-ERK, total ERK and GAPDH in lysate of HeLa cells co-transfected with Dr-EGFR and reporter plasmids and treated with light as in (a). (d) Quantification of lane intensities of phospho-ERK1/2 and total ERK, normalized to GAPDH represented as graphs. Left: downregulation of phospho-ERK1/2 upon action of 660 nm light (red line) and phospho-ERK1/2 downregulation in constantly activated with 780 nm light cells (black line). Right: total ERK1/2 levels. (e) Illumination pattern: inactivation of ERK signaling with 660 nm FR light was followed by 5 min 780 nm NIR stimulus; after that ERK signaling was inactivated with 660 nm light for 30 min. Samples were collected at 5, 10 and 30 min time points. (f) Illumination pattern for a negative control: inactivation of ERK signaling with 660 nm FR light was followed by 5 min 780 nm NIR light stimulus and by additional 30 min of 780 nm light. 25 μM BV was added to culture medium in all experiments. Error bars represent s.d., n=3 experiments.
