Abstract
Calderihabitans maritimus KKC1 is a thermophilic, carbon monoxide (CO)-utilizing, hydrogen-evolving bacterium that harbors seven cooS genes for anaerobic CO dehydrogenases and six hyd genes for [NiFe] hydrogenases and capable of using a variety of electron acceptors coupled to CO oxidation. To understand the relationships among these unique features and the transcriptional adaptation of the organism to CO, we performed a transcriptome analysis of C. maritimus KKC1 grown under 100% CO and N2 conditions. Of its 3114 genes, 58 and 32 genes were significantly upregulated and downregulated in the presence of CO, respectively. A cooS–ech gene cluster, an “orphan” cooS gene, and bidirectional hyd genes were upregulated under CO, whereas hydrogen-uptake hyd genes were downregulated. Transcriptional changes in anaerobic respiratory genes supported the broad usage of electron acceptors in C. maritimus KKC1 under CO metabolism. Overall, the majority of the differentially expressed genes were oxidoreductase-like genes, suggesting metabolic adaptation to the cellular redox change upon CO oxidation. Moreover, our results suggest a transcriptional response mechanism to CO that involves multiple transcription factors, as well as a CO-responsive transcriptional activator (CooA). Our findings shed light on the diverse mechanisms for transcriptional and metabolic adaptations to CO in CO-utilizing and hydrogen-evolving bacteria.
Electronic supplementary material
The online version of this article (10.1007/s00792-020-01175-z) contains supplementary material, which is available to authorized users.
Keywords: Carbon monoxide, Hydrogen, Hydrogenase, Energy conservation, Transcriptome, RNA-seq
Introduction
Carbon monoxide (CO) is used as an energy source by CO-oxidizing microbes (carboxydotrophs) because of its low redox potential (Ragsdale 2004; Oelgeschläger and Rother 2008; Sokolova et al. 2009; Diender et al. 2015). Carboxydotrophs harness CO dehydrogenases (CODHs) for CO utilization by catalyzing the reaction CO + H2O ⇌ CO2 + 2H+ + 2e− (Ragsdale 2004; Oelgeschläger and Rother 2008). CODHs are divided into two families: anaerobic Ni-containing CODHs (Ni-CODHs) and aerobic molybdenum- and copper-containing CODHs (Can et al. 2014; Hille et al. 2015). Unlike aerobic CODHs, Ni-CODHs can reduce ferredoxin and thereby utilize various types of terminal electron acceptors, such as protons, CO2, sulfate, and ferric iron [Fe(III)] (Oelgeschläger and Rother 2008; Sokolova et al. 2009; Diender et al. 2015). Because of this unique feature of Ni-CODHs, physiologically diverse anaerobic carboxydotrophs have been described, such as hydrogenogens, acetogens, methanogens, sulfate reducers, and Fe(III) reducers (Oelgeschläger and Rother 2008; Sokolova et al. 2009; Diender et al. 2015).
Hydrogenogenic carboxydotrophs couple CO oxidation with proton reduction to produce hydrogen (H2), during which the proton- or sodium-motive force is generated with residual energy via a Ni-CODH/energy converting hydrogenase (ECH) complex (Singer et al. 2006; Schut et al. 2016; Schoelmerich and Müller 2019). CO-dependent H2 production by hydrogenogenic carboxydotrophs is considered a “safety valve” to reduce toxic CO and supply H2, which is an energy source for H2-utilizing microbial communities (Techtmann et al. 2009). Hydrogenogenic carboxydotrophs are generally divided into three groups in their phylogeny: Firmicutes, Proteobacteria, and Archaea (Diender et al. 2015; Inoue et al. 2019). In Firmicutes, the Clostridia includes various types of thermophilic, hydrogenogenic carboxydotrophs that harbor multiple cooS genes and feature the Wood–Ljungdahl pathway (WLP) for carbon fixation (Techtmann et al. 2012; Shin et al. 2016; Inoue et al. 2019).
The functions of these cooS genes have been predicted from their genomic contexts such as ECH, WLP, and ferredoxin–NAD(P)H oxidoreductase (Techtmann et al. 2012; Inoue et al. 2019), which are presumed to be regulated by CO-responsive transcription factors, such as CooA and RcoM (Shelver et al. 1995; Komori et al. 2007; Kerby et al. 2008). However, recent studies of two hydrogenogenic carboxydotrophs, Carboxydothermus pertinax and Thermoanaerobacter kivui, show that the enzymatic coupling of cooS and ech genes that are distantly encoded in their respective genomes enables CO-dependent H2 production (Fukuyama et al. 2018, 2019a; Schoelmerich and Müller 2019). Moreover, cooS expression is upregulated in the presence of CO, despite the fact that no sequence motif is recognized by the CO-responsive transcriptional activator CooA in C. pertinax (Fukuyama et al. 2018, 2019a). Similarly, ech expression is upregulated in the presence of CO, although the genome does not encode any previously described CO-responsive transcription factors in T. kivui (Schoelmerich and Müller 2019). These studies suggest that there are previously unknown transcriptional response mechanisms to CO. Therefore, the direct observation of transcriptional, proteomic, and metabolic changes under CO is required to understand the metabolisms of hydrogenogenic carboxydotrophic bacteria.
Calderihabitans maritimus KKC1 is a thermophilic, obligately anaerobic, hydrogenogenic, carboxydotrophic bacterium in the Clostridia, closely related to Moorella, and was isolated from a core sample taken from marine sediment in the Kikai Caldera, Japan (Yoneda et al. 2013). C. maritimus KKC1 can grow anaerobically on 100% CO while producing H2 and CO2 with or without additional electron acceptors such as thiosulfate, sulfite, ferric citrate, amorphous Fe(III) oxide, Fe2O3, or fumarate, and on organic compounds, such as pyruvate, in the presence of thiosulfate. C. maritimus KKC1 requires yeast extract for CO-dependent growth unlike chemolithoautotrophic, hydrogenogenic carboxydotrophs, such as Moorella stamsii and Carboxydothermus hydrogenoformans, and is unable to grow with H2 and CO2 unlike chemolithoautotrophic acetogens, such as Moorella thermoacetica (Svetlichny et al. 1991; Drake and Daniel 2004; Alves et al. 2013; Yoneda et al. 2013). The draft genome of C. maritimus KKC1 encodes six cooS genes that are categorized by their genomic contexts, as follows: cooS1 (KKC1_RS04465), WLP; cooS2 (KKC1_RS06675), ECH; cooS3 (KKC1_RS06585), ferredoxin-NAD(P)H oxidoreductase; cooS4 (KKC1_RS12505), a cysteine synthase and ABC transporter; cooS5 (KKC1_RS04925), 2-oxoglutarate:ferredoxin oxidoreductase (Kor); and cooS6 (KKC1_RS10495), CooA (Omae et al. 2017). These six cooS genes harbor the complete sequence motifs that form three types of metal clusters for catalysis, although cooS1 is frame-shifted like other hydrogenogenic, carboxydotrophic Moorella and Carboxydothermus species possibly as a result of cultivation at high CO concentrations (Wu et al. 2005; Omae et al. 2017; Poehlein et al. 2018; Fukuyama et al. 2018, 2019b). To the best of our knowledge, the genome contains the highest number of cooS genes (Omae et al. 2017; Toshchakov et al. 2018) and encodes six hydrogenase gene clusters that include two ech gene clusters, a coo-type gene cluster (ech1, KKC1_RS06640–KKC1_RS06665) with cooS2, and a hyc/hyf-type gene cluster (ech2, KKC1_RS01155–KKC1_RS01200) with putative formate dehydrogenase genes (Omae et al. 2017). The genome only harbors one cooA gene as a CO-responsive transcription factor.
The multiplicity of Ni-CODHs and hydrogenases and the broad usage of electron acceptors in C. maritimus KKC1 suggest genomic adaptation for its carboxydotrophic growth (Yoneda et al. 2013; Omae et al. 2017); however, its metabolic and transcriptional responses to CO remain largely unknown. In this study, we performed a transcriptome analysis of C. maritimus KKC1 grown in the presence or absence of CO using RNA sequencing (RNA-seq). Under CO conditions, we found the evidence of transcriptional changes for Ni-CODHs and hydrogenases, and for anaerobic respiration, carbon and nitrogen metabolism, and transcription factors. We suggest that the transcriptional response mechanism to CO involves multiple transcription factors.
Materials and methods
Cultivation of C. maritimus KKC1
Calderihabitans maritimus KKC1 was cultured in modified NBRC 1251 medium, pH 7.5 at 65 °C under 100% CO or 100% N2 gas as previously described with slight modification (Yoneda et al. 2013). The medium contained 1 g sodium pyruvate, 1 g Na2S2O3·5H2O, 50 mg yeast extract, 16 g NaCl, 3.9 g MgSO4 ·7H2O, 3.9 g MgCl2·6H2O, 0.14 g CaCl2·2H2O, 0.65 g KCl, 0.5 g NaHCO3, 0.1 g NH4Cl, 0.1 g KH2PO4, 0.1 g NaBr, 10 mg Na2SiO3, 30 mg H3BO3, 15 mg SrCl2·6H2O, 6.6 mg FeCl3, 0.05 mg KI, 0.05 mg NaNO3, 10 mL trace mineral solution SL-4 (Pfennig and Lippert 1966), 1.0 mL vitamin solution (Wolin et al. 1963), 0.5 mg resazurin, and 0.1 g Na2S·9H2O per liter. Cultivation was performed in 100 mL of medium in a 250-mL bottle sealed with a rubber stopper and a polypropylene screw cap. The cells grown under 100% N2 gas were inoculated at 105–106 cells/mL into the fresh media under both 100% CO and 100% N2 gas conditions with three biological replicates. Cell growth was determined using an S3e Cell Sorter (Bio-Rad, Hercules, CA, USA) by counting the number of fluorescent signals from SYBR Green I-stained cells. Consumption of CO and evolution of H2 were analyzed by a GC-2014 gas chromatography system (Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector and a Shincarbon ST packed column (Shinwa Chemical Industries, Kyoto, Japan) using argon as the carrier gas. For RNA extraction, cells were collected on a 0.22-μm Durapore membrane filter (Merck Millipore, Burlington, MA, USA) at the late-exponential phase (Fig. 1a), and then stored in RNAlater RNA Stabilization Reagent (Qiagen, Hilden, Germany) at − 85 °C until RNA extraction.
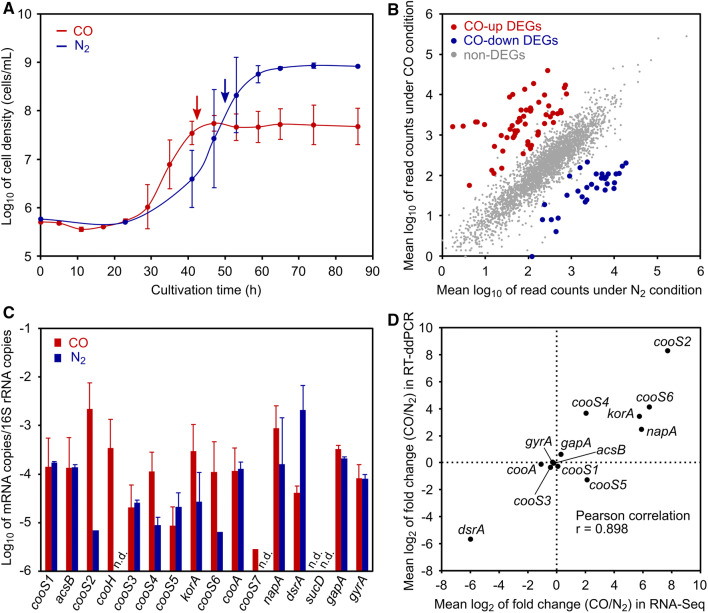
Fig. 1.
Overview of RNA-seq and RT-ddPCR datasets. a Growth curves of C. maritimus KKC1 in the presence (red) or absence (blue) of CO. The mean values of log10-transformed cell densities are plotted. Error bars indicate the standard deviation from three biological replicates. b Plots of read counts from genes in the presence or absence of CO. The mean values of log10-transformed read counts from two biological replicates are plotted. Upregulated DEGs in the presence of CO, downregulated DEGs, and non-DEGs are colored red, blue, and gray, respectively. c mRNA quantification by RT-ddPCR. The mean values of log10-transformed relative abundances of mRNA relative to 16S rRNA are shown. Values below the lower detection limit were omitted, although all samples were quantified with three biological replicates. Error bars indicate the standard deviation only when at least two values were above lower detection limit. d Comparison of fold-change values between RNA-seq and RT-ddPCR data. The mean values of log2-transformed fold changes of each gene are plotted. n.d. not detected
RNA isolation and cDNA synthesis
RNA isolation and cDNA synthesis were performed, as previously described (Fukuyama et al. 2018, 2019a). Total RNA was extracted using a mirVana miRNA Extraction Kit (Ambion, Austin, TX, USA), and the remaining genomic DNA was removed using TURBO DNase (Ambion). Total RNA was then purified using Agencourt RNAClean XP beads (Beckman Coulter, Brea, CA, USA), and quantified and quality controlled using a Qubit RNA HS Assay Kit (Invitrogen, Carlsbad, CA, USA) and an Agilent RNA 6000 Pico Kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). For RNA-seq analysis, rRNA was removed from the purified total RNA using a Ribo-Zero rRNA Removal Kit for Bacteria (Illumina, San Diego, CA, USA), and double-stranded cDNA was synthesized using a PrimeScript Double Strand cDNA Synthesis Kit (Takara, Shiga, Japan). For reverse transcriptase droplet digital polymerase chain reaction (RT-ddPCR), single-stranded cDNA was synthesized using a SuperScript III First-Strand Synthesis System (Invitrogen).
RNA-seq and data analysis
A DNA library for RNA-seq was prepared using a Nextera XT DNA Library Prep Kit (Illumina) with two biological replicates. The library was quantified and quality controlled using KAPA Library Quantification Kits (KAPA Biosystems, Wilmington, MA, USA) on a Thermal Cycler Dice Real Time System Single (Takara) and an Agilent High Sensitivity DNA Kit on an Agilent 2100 Bioanalyzer (Agilent Technologies). Sequencing was performed on the Illumina MiSeq instrument with an MiSeq Reagent Kit v3 (150 cycles), which generated 15,716,607 paired-end reads.
Quality filtering was performed using an FASTX-Toolkit version 0.0.14 (https://hannonlab.cshl.edu/fastx_toolkit/), with the reads being Q30 for > 80% of the bases. Reads from the remaining rRNA were removed using a BLASTn search (Camacho et al. 2009). The filtered reads were mapped onto the draft genome sequence (GCF_002207765.1) of C. maritimus KKC1 obtained from the National Centre for Biotechnology Information (NCBI) assembly database (NCBI Resource Coordinators 2018) using HISAT2 version 2.1.0 (Kim et al. 2015). The mapped data were checked using SAMtools version 0.1.19 (Li et al. 2009), and mapped reads per gene were counted using featureCounts from the Subread package version 1.6.3 (Liao et al. 2014). Normalization and differential expression analysis were performed using the R package edgeR version 3.22.5 (Robinson et al. 2009). Differentially expressed genes (DEGs) were identified using the functions “glmQLFit” and “glmTreat” with a fold-change significantly greater than 1.5 at a false discovery rate of ≤ 0.001. The read processing and data set statistics for differential expression analysis are summarized in Tables S1 and S2, respectively.
Functional annotation of the DEGs was performed automatically using EggNOG-mapper version 1.0.3 in HMMER mapping mode (Huerta-Cepas et al. 2017) and RAST server version 2.0 with RASTtk pipeline (Brettin et al. 2015). Annotation was manually confirmed using a BLASTp search (Camacho et al. 2009). Pathway analysis was performed using the Kyoto Encyclopedia of Genes and Genomes mapper version 3.2 (Kanehisa et al. 2012). N-terminal signal sequences were predicted using SignalP server version 5.0 (Almagro Armenteros et al. 2019), and hydrogenase annotation was performed using HydDB server (Søndergaard et al. 2016).
Prediction of transcriptional units and identification of transcription-factor-binding motifs
A unit of polycistronically transcribed genes, including DEGs, was manually predicted by checking the read alignments against the draft genome sequence of C. maritimus KKC1 using Integrative Genomics Viewer version 2.4.16 (Thorvaldsdóttir et al. 2013) (Table S3). The transcriptional unit comprised continuous same-stranded genes with continuous- and constant-read alignments. To discover sequence motifs in the upstream regions of DEGs, 300-bp upstream sequences from the start codon of the first gene in the transcriptional unit were curated from the genome sequence. Sequence motifs were searched using MEME version 4.12.0 (Bailey et al. 2009), and sequence logos were created using WebLogo version 2.8.2 (Crooks et al. 2004).
RT-ddPCR
To validate the RNA-seq data, RT-ddPCR was performed using a QX200 Droplet Digital PCR System (Bio-Rad) with three biological replicates. Each ddPCR mixture comprised 10 μL of 2 × ddPCR EvaGreen Supermix (Bio-Rad), 0.4 μL of 5 μM primer mix, and 1 μL of appropriately diluted cDNA in a final volume of 20 μL. Droplets were generated using a Droplet Generator (Bio-Rad) with 70 μL of Droplet Generator Oil for EvaGreen (Bio-Rad), and then transferred to a 96-well PCR plate (Eppendorf, Hamburg, Germany) and heat sealed. PCR amplification was performed on a TaKaRa PCR Thermal Cycler Dice Touch at 95 °C for 10 min followed by 40 cycles at 94 °C for 30 s and 58 °C for 60 s, one cycle at 98 °C for 10 min, and ending at 8 °C. After amplification, fluorescent signals from the droplets in each well were automatically measured using a Droplet Reader (Bio-Rad).
ddPCR data were analyzed using QuantaSoft software version 1.7.4 (Bio-Rad). To separate positive and negative droplets, thresholds of the fluorescent signals were manually set. The number of target DNA molecules in the reaction mixture was determined from the ratio of positive to total droplets. Data were quality controlled with > 10,000 total droplets in each sample and < 10 positive droplets in the negative control, except for the korA primers (< 20 positive droplets in the negative control). The lower limit of detection was set at > 10 positive droplets, except for the korA primers (> 20 positive droplets). To compare expression levels in different samples, concentrations of the target genes were normalized against the concentrations of 16S rRNA. All primers used for ddPCR were quality controlled with the lengths and melting curves of the products checked by agarose gel electrophoresis and real-time PCR, respectively (Table S4).
Data availability
The raw reads for RNA-Seq and the gene-expression profiles have been deposited in the NCBI/ENA/DDBJ Sequence Read Archive under accession number DRA008406.
Results
Overview of RNA-seq analysis
We performed RNA-seq experiments for late-exponential cultures of C. maritimus KKC1 grown under 100% CO and N2 gas conditions in biological duplicates (Fig. 1a). To enable growth under N2 conditions, pyruvate and thiosulfate were added to the medium under both gas conditions. Under CO conditions, conversion of ~ 10% of CO to H2 was observed at the sampling point by gas chromatography (data not shown). Over 3 million high-quality RNA-seq reads in each sample were mapped with sufficient coverages (> 100 × mean coverage in each sample) and mapping efficiencies (> 80% in each sample) (Table S1). The biological replicates for the CO and N2 conditions of the RNA-seq experiments were highly reproducible with Pearson’s correlation coefficients of 0.968 and 0.970, respectively (Fig. S1).
Normalization and differential gene-expression analysis were performed using mapped RNA-seq data. Of the 3,114 genes in C. maritimus KKC1, 90 were differentially expressed between the two conditions, with these DEGs including 58 upregulated and 32 downregulated genes in the presence of CO and log2-fold changes for CO/N2 ranging from 2.57 to 10.3 and from − 7.16 to − 2.68, respectively (Fig. 1b and Table S2). Approximately 60% of the upregulated and ~ 70% of the downregulated DEGs in the presence of CO were grouped into the “metabolism” categories of Clusters of Orthologous Groups (COGs) (Galperin et al. 2015), with > 40% of both upregulated and downregulated DEGs were grouped in the COG functional category “C” (energy production and conversion) (Table S2). These data are comparable with those obtained by a recent RNA-seq study of C. pertinax (Fukuyama et al. 2019a).
The expression levels of the seven DEGs and eight non-DEGs, including cooS genes (cooS1 through cooS6) and house-keeping genes (gapA and gyrA), were confirmed by RT-ddPCR (Fig. 1c). The C. maritimus KKC1genome also contains two partial cooS-like gene fragments (KKC1_RS14835 and KKC1_RS01100) (Omae et al. 2017) sharing 65 bp and 16 aa perfect matches encoding one protein (hereafter designated as CooS7). The expression level of cooS7 judged as non-DEGs was also tested. The fold-change values in RNA-seq and RT-ddPCR data were highly correlated, with a Pearson’s correlation coefficient of 0.898 (Fig. 1d), suggesting that our RNA-seq data captured the precise transcriptional changes between CO and N2 conditions.
CO-dependent differential expression of multiple genes encoding Ni-CODHs and hydrogenases
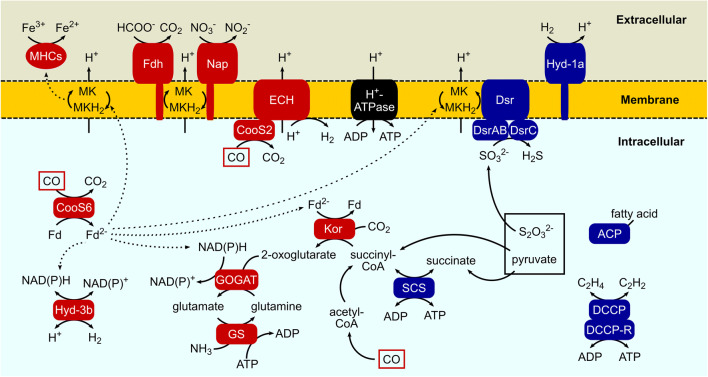
We first focused on transcriptional changes in Ni-CODHs and hydrogenases, which are responsible for carboxydotrophic and hydrogenogenic growth. Of the seven cooS genes in C. maritimus KKC1, only cooS2 and cooS6 were identified as upregulated DEGs in the presence of CO (Fig. 2; Tables 1 and S2). All of the genes in the cooS2–ech1 (coo-type) gene cluster (KKC1_RS06640–KKC1_RS06680) were upregulated DEGs in the presence of CO, indicating that the Ni-CODH/ECH (Coo-type) complex was responsible for CO-dependent H2 production, similar to other hydrogenogenic carboxydotrophs (Soboh et al. 2002; Singer et al. 2006; Schut et al. 2016; Schoelmerich and Müller 2019). Conversely, the expression level of the ech2 (hyc/hyf-type) gene cluster (KKC1_RS01155–KKC1_RS01200) was unchanged under CO conditions, indicating a potential function with its gene neighbor encoding the putative formate dehydrogenase.
Fig. 2.
Schematic representation of metabolic pathways in C. maritimus KKC1 with DEGs. Only pathways discussed in the text are shown. Protein machineries with upregulated and downregulated DEGs in the presence of CO are colored red and blue, respectively. ATP synthase (H+-ATPase) is colored black. Dotted arrows indicate the possible pathways of electron flow from CO oxidation. MK menaquinone, MKH2 menaquinol
Table 1.
List of Ni-CODHs and hydrogenases identified in DEGs
| Functional group | Locus tag | Log2FC | Annotation | COG number (category)a |
|---|---|---|---|---|
| CooS2/Ech1 | KKC1_RS06640 | 8.4 | CooM | COG0651,COG1007 (C) |
| CooS2/Ech1 | KKC1_RS06645 | 7.9 | CooK | COG0650 (C) |
| CooS2/Ech1 | KKC1_RS06650 | 9.5 | CooL | COG3260 (C) |
| CooS2/Ech1 | KKC1_RS06655 | 8.8 | CooX | COG1143 (C) |
| CooS2/Ech1 | KKC1_RS06660 | 10.3 | CooU | (C) |
| CooS2/Ech1 | KKC1_RS06665 | 9.0 | CooH | COG3261 (C) |
| CooS2/Ech1 | KKC1_RS06670 | 8.5 | CooF | COG1142 (C) |
| CooS2/Ech1 | KKC1_RS06675 | 7.7 | CooS | COG1151 (C) |
| CooS2/Ech1 | KKC1_RS06680 | 6.0 | CooC | COG3640 (D) |
| CooS6 | KKC1_RS10495 | 6.5 | CooS | COG1151 (C) |
| Hyd-3b | KKC1_RS10615 | 5.0 | Group 3b Ni,Fe-hydrogenase beta subunit | COG1145 (C) |
| Hyd-3b | KKC1_RS10620 | 5.0 | Group 3b Ni,Fe-hydrogenase gamma subunit | COG0543 (C) |
| Hyd-3b | KKC1_RS10625 | 4.8 | Group 3b Ni,Fe-hydrogenase small subunit | COG1941 (C) |
| Hyd-3b | KKC1_RS10630 | 5.8 | Group 3b Ni,Fe-hydrogenase large subunit | COG3259 (C) |
| Hyd-3b | KKC1_RS10635 | 4.8 | Ni,Fe-hydrogenase maturation factor | (O) |
| Hyd-1a | KKC1_RS00385 | − 5.2 | Group 1a Ni,Fe-hydrogenase small subunit | COG1740 (C) |
| Hyd-1a | KKC1_RS00390 | − 4.1 | Group 1a Ni,Fe-hydrogenase large subunit | COG0374 (C) |
| Hyd-1a | KKC1_RS00395 | − 4.8 | Group 1a Ni,Fe-hydrogenase cytochrome b subunit | COG1969 (C) |
| Hyd-1a | KKC1_RS00400 | − 4.1 | Ni,Fe-hydrogenase maturation factor | COG0680 (O) |
aCOG number and category were annotated by EggNOG mapper. Note that some COG categories were annotated, even when COG numbers were not annotated
FC fold change
cooS6 expression was upregulated, whereas that of an adjacent upstream gene (cooA) was unchanged, and the other five cooS genes were not identified as DEGs (Figs. 1d and 2; Table 1 and S2). It should be noted that the expression level of cooS4 was higher in the presence of CO in both RNA-Seq and RT-ddPCR experiments albeit a non-DEG (Fig. 1c and d; Table S2). RNA-Seq and RT-ddPCR revealed that cooS1 and acsB, which are involved in WLP, were highly expressed in the presence and absence of CO (although cooS1 is frame-shifted) (Fig. 1c and Table S2), similar to results from recent RNA-seq data for C. pertinax (Fukuyama et al. 2019a). These data suggested that gene transcription for WLP is unchanged between CO and N2 conditions in these hydrogenogenic, carboxydotrophic bacteria.
Two of the four putative [NiFe] hydrogenase genes other than the two ech genes in C. maritimus KKC1 were identified as DEGs (Fig. 2; Table 1 and S2). Under CO conditions, the expression levels of hyd-3b genes (KKC1_RS10620–KKC1_RS10635) encoding group 3b [NiFe] hydrogenase catalytic subunits and their maturation protease were upregulated, whereas those of hyd-1a genes (KKC1_RS00385–KKC1_RS00400) encoding group 1a [NiFe] hydrogenase catalytic subunits, a cytochrome b subunit, and their maturation protease were downregulated. The group 3b [NiFe] hydrogenase is a cytoplasmic, bidirectional hydrogenase that directly couples NADPH oxidation to H2 evolution (Greening et al. 2016; Søndergaard et al. 2016), although it remains unclear whether NADPH is utilized by this enzyme in C. maritimus KKC1. Conversely, the group 1a [NiFe] hydrogenase is a periplasmic, unidirectional H2-uptake hydrogenase, and the cytochrome b subunit is considered a putative electron carrier, as are other group 1 hydrogenases (Greening et al. 2016; Søndergaard et al. 2016); however, a terminal electron acceptor has not been identified in C. maritimus KKC1.
Transcriptional changes in anaerobic respiration systems under CO conditions
Other than Ni-CODHs and hydrogenases, anaerobic respiration genes required for energy production and conversion were identified as DEGs. In the presence of CO, the expression levels of gene clusters encoding periplasmic formate dehydrogenase (Fdh)-like proteins (KKC1_RS05300–KKC1_RS05315) and periplasmic nitrate reductase (Nap)-like proteins (KKC1_RS14135–KKC1_RS14170) were significantly upregulated, whereas those of a gene cluster encoding dissimilatory sulfite reductase (Dsr)-like proteins (KKC1_RS04115–KKC1_RS04185 and KKC1_RS04195) were downregulated (Fig. 2; Table 2 and S2). The Dsr complex couples sulfite reduction with a proton-translocating menaquinone cycle (Venceslau et al. 2014; Santos et al. 2015; Anantharaman et al. 2018), whereas Fdh and Nap couple formate oxidation with nitrate reduction to drive the menaquinone cycle (Fig. 2) (Richardson et al. 2004; Cerqueira et al. 2015). Under N2 conditions, energy production is mainly performed through pyruvate oxidation and thiosulfate reduction (Yoneda et al. 2013). Our data suggested that the Dsr complex catalyzes electron transfer to the menaquinone pool by reducing the sulfite derived from thiosulfate in the absence of CO. Conversely, in the presence of CO, both Fdh- and Nap-like proteins would maintain the menaquinone cycle instead of Dsr, although authentic substrates of these proteins have not been identified.
Table 2.
List of anaerobic respiration machineries identified in DEGs
| Functional group | Locus tag | Log2FC | Annotation | COG number (category)a |
|---|---|---|---|---|
| Fdh | KKC1_RS05300 | 3.1 | Twin-arginine translocase TatA/TatE family subunit | (U) |
| Fdh | KKC1_RS05305 | 3.7 | FdoI | COG2864 (C) |
| Fdh | KKC1_RS05310 | 3.4 | FdoH | COG0437 (C) |
| Fdh | KKC1_RS05315 | 3.7 | FdoG | COG0243 (C) |
| Nap | KKC1_RS14135 | 5.8 | Twin-arginine translocase TatA/TatE family subunit | (U) |
| Nap | KKC1_RS14140 | 5.8 | NapD | |
| Nap | KKC1_RS14145 | 5.7 | NapB | |
| Nap | KKC1_RS14150 | 5.9 | NapG | COG1145 (C) |
| Nap | KKC1_RS14155 | 5.9 | NapA | COG0243 (C) |
| Nap | KKC1_RS14160 | 5.7 | Hypothetical protein (pseudogene) | |
| Nap | KKC1_RS14165 | 5.8 | NapH | COG0348 (C) |
| Nap | KKC1_RS14170 | 5.8 | NapG | COG1145 (C) |
| MHCs | KKC1_RS04440 | 7.7 | MHC-1 (12 × CX2H motifs) | (S) |
| MHCs | KKC1_RS08310 | 7.9 | MHC-2 (7 × CX2H motifs) | (S) |
| MHCs | KKC1_RS11980 | 3.2 | MHC-3 (12 × CX2H motifs) | (C) |
| Dsr | KKC1_RS04115 | – 6.0 | DsrT | (S) |
| Dsr | KKC1_RS04120 | – 5.7 | DsrK | |
| Dsr | KKC1_RS04125 | – 6.1 | DsrJ | (C) |
| Dsr | KKC1_RS04130 | – 5.0 | DsrO | COG0437 (C) |
| Dsr | KKC1_RS04135 | – 5.8 | DsrP | COG5557 (C) |
| Dsr | KKC1_RS04140 | – 5.5 | DsrM | COG2181 (C) |
| Dsr | KKC1_RS04145 | – 6.0 | DsrA | COG2221 (C) |
| Dsr | KKC1_RS04150 | – 6.1 | DsrB | COG2221 (C) |
| Dsr | KKC1_RS04155 | – 6.7 | DsrD | (S) |
| Dsr | KKC1_RS04160 | – 6.6 | Ferredoxin | (C) |
| Dsr | KKC1_RS04165 | – 7.2 | DsrC | COG2920 (P) |
| Dsr | KKC1_RS04170 | – 5.1 | Pyridine nucleotide-disulphide oxidoreductase | COG0446,COG0607 (P) |
| Dsr | KKC1_RS04175 | – 5.3 | DsrN | COG1797 (H) |
| Dsr | KKC1_RS04180 | – 6.2 | DsrM | COG2181 (C) |
| Dsr | KKC1_RS04185 | – 6.1 | DsrK | COG0247 (C) |
| Dsr | KKC1_RS04195 | – 2.8 | DsrE | COG1416 (S) |
aCOG number and category were annotated by EggNOG mapper. Note that some COG categories were annotated, even when COG numbers were not annotated
FC fold change
Additionally, we found that the upregulated DEGs included three genes encoding the putative extracellular multiheme cytochromes c (MHCs; mhc-1, mhc-2, and mhc-3, corresponding to KKC1_RS04440, KKC1_RS08310, and KKC1_RS11980, respectively), which are responsible for redox cycles of metals including Fe(III), nitrogen compounds, and sulfur compounds by catalyzing electron transfer from/to quinone pools in the extracellular space (Mowat and Chapman 2005; Zhong and Shi 2018; Chong et al. 2018) (Fig. 2; Table 2 and S2). MHC-1, MHC-2, and MHC-3 have 12, 7, and 12 CX2CH motifs capable of binding a heme moiety, respectively, and all three proteins were predicted to have N-terminal signal sequences for extracellular localization, indicating their involvement in extracellular electron transfer.
Carbon and nitrogen metabolism as a possible redox-buffering system in the CO response
Reducing equivalents from CO can be utilized for other redox metabolic pathways. In the presence of CO, genes encoding Kor-like proteins (KKC1_RS04895–KKC1_RS04910), a glutamine synthetase (GS)-like protein (KKC1_RS05230), and glutamate synthase (GOGAT)-like proteins (KKC1_RS05235–KKC1_RS05240) were identified as upregulated DEGs (Fig. 2; Table 3 and S2). Kor catalyzes the reversible conversion of succinyl-CoA to 2-oxoglutarate through (de)carboxylating reactions, using ferredoxin as an electron carrier (Fig. 2) (Yamamoto et al. 2010; Li and Elliott 2016; Chen et al. 2019). GOGAT produces two molecules of glutamate from 2-oxoglutarate and glutamine using NAD(P)H (Vanoni and Curti 2005), whereas GS produces glutamine from glutamate and ammonia using ATP (Eisenberg et al. 2000). It should be noted that a gene encoding a succinyl-CoA synthetase (SCS) μ subunit (KKC1_RS08680) was significantly downregulated in the presence of CO (Fig. 2; Table 3 and S2); however, C. maritimus KKC1 possesses three SCS gene sets, and the expression levels of the other two SCS genes were unchanged under CO conditions (Table S2).
Table 3.
List of DEGs related to carbon and nitrogen metabolisms
| Functional group | Locus tag | Log2FC | Annotation | COG number (category)a |
|---|---|---|---|---|
| Kor | KKC1_RS04895 | 6.0 | KorD | (C) |
| Kor | KKC1_RS04900 | 5.8 | KorA | COG0674 (C) |
| Kor | KKC1_RS04905 | 5.8 | KorB | COG1013 (C) |
| Kor | KKC1_RS04910 | 5.0 | KorG | COG1014 (C) |
| GS/GOGAT | KKC1_RS05230 | 3.8 | Glutamine synthetase | COG0174 (E) |
| GS/GOGAT | KKC1_RS05235 | 4.3 | Glutamate synthase domain 1 | COG0067 (E) |
| GS/GOGAT | KKC1_RS05240 | 3.3 | Glutamate synthase domain 2 | COG0069 (E) |
| SCS | KKC1_RS08680 | − 6.4 | Succinyl-CoA synthetase subunit alpha | COG0074 (C) |
| ACP | KKC1_RS01055 | − 2.7 | Acyl carrier protein | COG0236 (I) |
| DCCP | KKC1_RS14820 | − 5.5 | DCCP | COG1775 (E) |
| DCCP | KKC1_RS14825 | − 4.5 | DCCP reductase | COG1924 (I) |
aCOG number and category were annotated by EggNOG mapper. Note that some COG categories were annotated, even when COG numbers were not annotated
FC fold change
Genes for a double-cubane cluster protein (DCCP), DCCP reductase (KKC1_RS14820–KKC1_RS14825), and an acyl carrier protein (KKC1_RS01055) were also identified as downregulated DEGs under CO conditions (Fig. 2; Table 3 and S2). DCCP and DCCP reductase represent a recently identified, novel, ATP-dependent oxidoreductase system in C. hydrogenoformans (Jeoung and Dobbek 2018) and the amino acid identities of DCCP and DCCP reductase homologs of C. maritimus KKC1 to those of C. hydrogenoformans were 57% and 41%, respectively. DCCP and DCCP reductase reduce acetylene to ethylene, with this activity inhibited by CO. Additionally, the acyl carrier protein plays a central role in fatty-acid biosynthesis, which requires the NADPH-dependent reduction of an acyl chain (Chan and Vogel 2010).
Transcription factors as DEGs
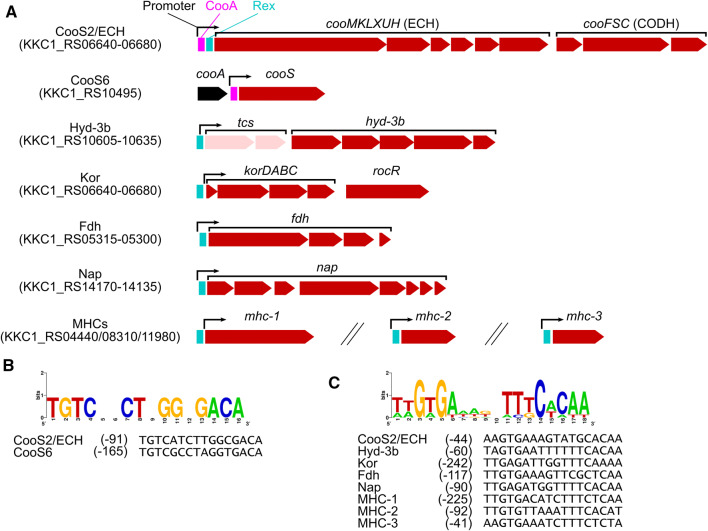
Putative transcription factors were also identified among the DEGs. Genes encoding two RocR-like AAA+ superfamily transcriptional activators (KKC1_RS02905 and KKC1_RS04915) and one TetR/AcrR family transcriptional regulator (KKC1_RS07140) were upregulated in the presence of CO, whereas an LysR family transcriptional regulator (KKC1_RS14830) was downregulated (Table 4 and S2). To analyze the relationships between these transcription factors and their targets, the transcriptional units were predicted using the mapped RNA-seq data that included DEGs (Table S3). One gene encoding an RocR-like protein (KKC1_RS04915) was adjacent to the kor genes, which were significantly upregulated, whereas the other gene (KKC1_RS02905) was an orphan. These two proteins shared 89% amino acid identity and possessed a conserved HX2CX2CX5C motif, possibly used for metal binding in the N-terminal sensor domain. RocR-like proteins were originally positive regulators of arginine catabolism in bacteria (Calogero et al. 1994). Genes encoding a TetR/AcrR-like protein and a LysR-like protein were located at transcriptional units encoding efflux transporter systems and DCCP/DCCP reductase, respectively (Tables S2 and S3). Moreover, our prediction of the transcriptional units revealed that two-component-system-like genes (KKC1_RS10605–KKC1_RS10610) were located in the unit containing hyd-3b genes, although these two-component-system-like genes were not identified as DEGs (Fig. 3a; Tables S2 and S3). These transcription factors might alter DEG expression levels in C. maritimus KKC1.
Table 4.
List of transcription factors in DEGs
| Functional group | Locus tag | Log2FC | Annotation | COG number (category)a |
|---|---|---|---|---|
| RocR | KKC1_RS02905 | 3.3 | RocR-like transcriptional activator | COG3829 (K) |
| RocR | KKC1_RS04915 | 4.7 | RocR-like transcriptional activator | COG3829 (K) |
| TetR | KKC1_RS07140 | 2.8 | TetR/AcrR family transcriptional regulator | (K) |
| LysR | KKC1_RS14830 | − 2.9 | LysR family transcriptional regulator | COG0583 (K) |
aCOG number and category were annotated by EggNOG mapper. Note that some COG categories were annotated, even when COG numbers were not annotated
FC fold change
Fig. 3.
Prediction of transcriptional response to CO in C. maritimus KKC1. a Predicted structure of CO-responsive transcriptional units regulated by CooA and Rex. The predicted promoter regions (− 10 and − 35 sequences) are indicated by arrows. Regions of sequence motifs recognized by CooA and Rex are colored magenta and cyan, respectively. Upregulated DEGs in the presence of CO and upregulated non-DEGs are colored red and pale red, respectively. The cooA gene, which is upstream of cooS6, is shown in black. tcs, two-component system genes. b,c Putative b CooA- and c Rex-binding sequence motifs. The sequence logo is shown above each sequence alignment, and the number of bases from the start codon is shown in parentheses
Transcription factor-binding motifs
To identify transcription-factor-binding motifs in the upstream regions of the DEGs, we searched for palindromic sequence motifs in the predicted transcriptional units (Table S3). Putative sequence motifs recognized by a CO-sensing transcriptional activator CooA and a redox-sensing transcriptional regulator Rex were identified in the transcriptional units upregulated by CO, whereas no transcription-factor-binding motif was identified in the downregulated transcriptional units (Fig. 3). A CooA-binding consensus sequence (5′-TGTC-N8-GACA) was found in the upstream regions of the cooS2–ech1 gene cluster and cooS6, as previously reported (Fig. 3a and b) (Omae et al. 2017), whereas Rex-binding sequence (5′-TTGTGA-N6-TCACAA)-like motifs were found in the upstream regions of the transcriptional units containing the cooS2–ech1, hyd-3b, kor, fdh, and nap gene clusters and three mhc genes (Fig. 3a and c). These data implied that CooA and Rex are involved in the upregulation of these genes in the presence of CO in situations involving direct CO sensing and redox sensing, respectively.
Discussion
We examined transcriptomic changes in C. maritimus KKC1 growing in the presence or absence of CO using RNA-seq analysis. Our data showed that of the seven cooS genes and six hydrogenase genes studied, the cooS2–ech1 (coo-type) gene cluster (not the hyc/hyf-type gene cluster), the “orphan” cooS6 gene, and bidirectional hyd-3b genes were upregulated under CO condition (Fig. 2 and Table 1). In hydrogenogenic carboxydotrophic Moorella species, genes for the hyc/hyf-type hydrogenase form a gene cluster with cooS and are responsible for CO-dependent H2 production (Poehlein et al. 2018; Fukuyama et al. 2019b), while in Carboxydothermus species, the coo-type works with cooS (Wu et al. 2005; Fukuyama et al. 2019a), suggesting that C. maritimus KKC1 utilizes a strategy similar to Carboxydothermus species rather than Moorella species for CO-dependent energy conservation. Upregulation of the “orphan” cooS6 gene under CO conditions could induce the reduction of the ferredoxin pool and perturbation of cellular redox balance in C. maritimus KKC1. The bidirectional hyd-3b genes might balance such redox perturbation. Moreover, unlike C. pertinax, H2-uptake hyd-1a genes were downregulated under CO conditions in C. maritimus KKC1 (Fig. 2 and Table 1) (Fukuyama et al. 2019a). These data suggest that an H2-evolution system is predominant in the presence of CO because of the excessive reducing equivalents from CO in C. maritimus KKC1.
Our data support the broad usage of electron acceptors in anaerobic respiration of C. maritimus KKC1 (Fig. 2 and Table 2). We found that three mhc genes presumed to utilize Fe(III) were upregulated in the presence of CO. In Thermincola potens, MHCs are responsible for respiratory electron transfer to Fe(III) (Carlson et al. 2012), and genomic analysis of Carboxydocella thermoautotrophica indicated the possible involvement of MHCs in CO-dependent Fe(III) reduction (Toshchakov et al. 2018). Similar to these hydrogenogenic carboxydotrophs, C. maritimus KKC1 produces Fe(II) with ferric citrate, amorphous Fe(III) oxide, and Fe2O3 in the presence of CO, suggesting that Fe(III) is used as an electron acceptor under CO conditions (Yoneda et al. 2013). Therefore, these MHCs might be responsible for extracellular electron transfer to Fe(III) in C. maritimus KKC1. On the contrary, dsr genes that utilize sulfite in thiosulfate respiration were downregulated in the presence of CO although C. maritimus KKC1 is considered to couple thiosulfate reduction with CO or pyruvate oxidation (Yoneda et al. 2013). Thiosulfate respiration is conducted by a concerted way of thiosulfate/polysulfide reductase (Phs) and Dsr (Stoffels et al. 2011; Venceslau et al. 2014). We could not identify any phs genes in C. maritimus KKC1; therefore, a complete thiosulfate reduction pathway in this organism remains unknown. C. pertinax, which can also utilize Fe(III) and thiosulfate with CO or pyruvate oxidation, shows that expressions of the mhc-like genes, the dsr gene cluster (cpu_17430–17,360), and the phs gene cluster (cpu_06910–06,930) remain unchanged between CO and N2 conditions (Fukuyama et al. 2019a), suggesting that transcriptional responses in the anaerobic respiration pathway upon CO would differ between these two hydrogenogenic carboxydotrophs.
Oxidoreductase-like genes encoding Kor, GOGAT, and GS involved in carbon and nitrogen metabolisms were also upregulated under CO conditions in C. maritimus KKC1, strongly suggesting metabolic adaptation to cellular redox change upon CO oxidation (Fig. 2 and Table 3). Moreover, kor genes are upregulated in Thermococcus onnurineus under CO conditions, suggesting the possible involvement of Kor in carbon fixation (Moon et al. 2012), whereas the GS/GOGAT cycle is a redox-buffering system involving the consumption of NAD(P)H in Caldicellulosiruptor bescii and Clostridium thermocellum (Sander et al. 2015, 2019). It is possible that the enzymatic cycle involving Kor, GOGAT, and GS would assimilate carbon and nitrogen using excessive reducing equivalents from CO.
Additionally, we identified putative transcription factors as DEGs (Table 4) and putative CooA- and Rex-binding motifs in the upstream regions of upregulated DEGs (Fig. 3). As noted, the expression of cooA remained unchanged under CO conditions, suggesting that the basal expression level of cooA would be adequate to adapt to CO (Table S2). This phenomenon is different in C. pertinax and Desulfovibrio vulgaris, where cooA genes are upregulated in the presence of CO (Rajeev et al. 2012; Fukuyama et al. 2019a). Moreover, Rex is a redox-sensing transcriptional repressor and conserved among various bacteria, regardless of whether they are anaerobes or aerobes, and conformational changes from NAD+- to NADH-bound states induce transcription by releasing Rex from its recognition sequence (McLaughlin et al. 2010; Ravcheev et al. 2012). C. maritimus KKC1 possesses one Rex homolog (KKC1_RS10865), which exhibits 39% and 41% amino acid identities with those from C. bescii and Clostridium acetobutylicum, respectively. As described here, C. bescii Rex regulates various types of hydrogenases including ECH, and pyruvate:ferredoxin oxidoreductase belonging to the same family as Kor (Sander et al. 2019). In Clostridium species, Rex regulates the expressions of genes encoding various types of oxidoreductase, including nitrate reductases, hydrogenases, and WLP enzymes (Zhang et al. 2014). Therefore, cellular redox changes under CO conditions in C. maritimus KKC1 might result in a high NADH/NAD+ ratio to drive Rex-dependent transcriptional activation of oxidoreductase-like genes.
These findings suggest a possible multi-step transcriptional response to CO in C. maritimus KKC1 as follow: 1) upon CO exposure, CO-sensing CooA activates transcription of the cooS2–ech1 gene cluster and cooS6, and these two Ni-CODHs catalyze CO oxidation to supply reducing equivalents within the cell; 2) excessive reducing equivalents from CO result in a high NADH/NAD+ ratio, followed by Rex-dependent transcriptional activation of the cooS2–ech1, hyd-3b, kor, fdh, and nap gene clusters, and three mhc genes; and 3) changes in the metabolisms or expression of transcription factors induce alterations in the transcription of other DEGs.
Our data highlight the diversity of CO-responsive transcriptional regulation in thermophilic, hydrogenogenic, carboxydotrophic bacteria. In C. pertinax, of the nine gene clusters upregulated in the presence of CO, including cooS, only the ech gene cluster is directly regulated by CooA (Fukuyama et al. 2019a), whereas the Rex-binding motif or those of other transcription factors are not found in upstream regions of these nine gene clusters. Additionally, in hydrogenogenic, carboxydotrophic Moorella strains, whose genomes encode no known CO-sensing transcription factor homologs, genes for RocR-like transcriptional activators (MOST_RS16225 in M. stamsii and MOTE_RS04420 in M. thermoacetica DSM 21,394) are located in the upstream regions of their Ni-CODH–ECH gene clusters. Because two genes for RocR-like proteins were upregulated under CO condition in C. maritimus KKC1 (Table 4), RocR-like proteins related to Ni-CODH–ECH gene clusters in these two Moorella species might be involved in response to CO. Moreover, a recent comparative genomics study of Parageobacillus thermoglucosidasius, a hydrogenogenic carboxydotroph also lacking known CO-sensing transcription factors, has found a transition-state regulator Hpr-binding sequence in the upstream region of its Ni-CODH–ECH gene cluster (Mohr et al. 2018). These findings imply previously undescribed transcriptional response mechanisms to CO. There could be various ways to respond to CO, including directly sensing CO, via stress caused by CO, or through cellular redox or metabolic changes via CO oxidation. Therefore, the diverse strategies for adaptation to CO-dependent metabolism would have been evolved in thermophilic, hydrogenogenic, carboxydotrophic bacteria.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Computation time was provided by the SuperComputer System, Institute for Chemical Research, Kyoto University. The work was supported by JSPS KAKENHI Grant Number JP16H06381 (to Y.S.). M.I., T.Y., and Y.S. conceived and designed the study. M.I. and H.I. performed the RNA-seq and RT-ddPCR experiments and data analysis. M.I., T.Y., and Y.S. wrote the manuscript with the assistance of H.I., Y.F., and K.O. All of the authors reviewed and approved the manuscript.
Abbreviations
- CODH
Carbon monoxide dehydrogenase
- Ni-CODH
Anaerobic Ni-containing CODH
- ECH
Energy converting hydrogenase
- WLP
Wood–Ljungdahl pathway
- Kor
2-Oxoglutarate:ferredoxin oxidoreductase
- RNA-seq
RNA sequencing
- RT-ddPCR
Reverse transcriptase droplet digital polymerase chain reaction
- NCBI
The National Centre for Biotechnology Information
- DEG
Differentially expressed gene
- COG
Cluster of orthologous groups
- Fdh
Periplasmic formate dehydrogenase
- Nap
Periplasmic nitrate reductase
- Dsr
Dissimilatory sulfite reductase
- MHC
Extracellular multiheme cytochromes c
- GS
Glutamine synthetase
- GOGAT
Glutamate synthase
- SCS
Succinyl-CoA synthetase
- DCCP
Double-cubane cluster protein
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almagro Armenteros JJ, Tsirigos KD, Sønderby CK, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37:420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- Alves JI, van Gelder AH, Alves MM, et al. Moorella stamsii sp. nov., a new anaerobic thermophilic hydrogenogenic carboxydotroph isolated from digester sludge. Int J Syst Evol Microbiol. 2013;63:4072–4076. doi: 10.1099/ijs.0.050369-0. [DOI] [PubMed] [Google Scholar]
- Anantharaman K, Hausmann B, Jungbluth SP, et al. Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle. ISME J. 2018;12:1715–1728. doi: 10.1038/s41396-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettin T, Davis JJ, Disz T, et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep. 2015;5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero S, Gardan R, Glaser P, et al. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J Bacteriol. 1994;176:1234–1241. doi: 10.1128/jb.176.5.1234-1241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, et al. BLAST+: Architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can M, Armstrong FA, Ragsdale SW. Structure, function, and mechanism of the nickel metalloenzymes, CO dehydrogenase, and acetyl-CoA synthase. Chem Rev. 2014;114:4149–4174. doi: 10.1021/cr400461p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson HK, Iavarone AT, Gorur A, et al. Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram-positive bacteria. Proc Natl Acad Sci USA. 2012;109:1702–1707. doi: 10.1073/pnas.1112905109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira NMFSA, Gonzalez PJ, Fernandes PA, et al. Periplasmic nitrate reductase and formate dehydrogenase: similar molecular architectures with very different enzymatic activities. Acc Chem Res. 2015;48:2875–2884. doi: 10.1021/acs.accounts.5b00333. [DOI] [PubMed] [Google Scholar]
- Chan DI, Vogel HJ. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem J. 2010;430:1–19. doi: 10.1042/BJ20100462. [DOI] [PubMed] [Google Scholar]
- Chen PYT, Li B, Drennan CL, Elliott SJ. A reverse TCA cycle 2-oxoacid: ferredoxin oxidoreductase that makes C-C bonds from CO2. Joule. 2019;3:595–611. doi: 10.1016/j.joule.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong GW, Karbelkar AA, El-Naggar MY. Nature’s conductors: what can microbial multi-heme cytochromes teach us about electron transport and biological energy conversion? Curr Opin Chem Biol. 2018;47:7–17. doi: 10.1016/j.cbpa.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A Sequence Logo Generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diender M, Stams AJM, Sousa DZ. Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front Microbiol. 2015;6:1275. doi: 10.3389/fmicb.2015.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake HL, Daniel SL. Physiology of the thermophilic acetogen Moorella thermoacetica. Res Microbiol. 2004;155:869–883. doi: 10.1016/j.resmic.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Gill HS, Pfluegl GMU, Rotstein SH. Structure-function relationships of glutamine synthetases. Biochim Biophys Acta - Protein Struct Mol Enzymol. 2000;1477:122–145. doi: 10.1016/S0167-4838(99)00270-8. [DOI] [PubMed] [Google Scholar]
- Fukuyama Y, Omae K, Yoneda Y, et al. Insight into energy conservation via alternative carbon monoxide metabolism in Carboxydothermus pertinax revealed by comparative genome analysis. Appl Environ Microbiol. 2018;84:e00458-18. doi: 10.1128/aem.00458-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama Y, Omae K, Yoshida T, Sako Y. Transcriptome analysis of a thermophilic and hydrogenogenic carboxydotroph Carboxydothermus pertinax. Extremophiles. 2019;23:389–398. doi: 10.1007/s00792-019-01091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama Y, Tanimura A, Inoue M, et al. Draft genome sequences of two Thermophilic Moorella sp. strains, isolated from an acidic hot spring in Japan. Microbiol Resour Announc. 2019;8:e00663-19. doi: 10.1128/MRA.00663-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY, Makarova KS, Wolf YI, Koonin EV. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015;43:D261–D269. doi: 10.1093/nar/gku1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening C, Biswas A, Carere CR, et al. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016;10:761–777. doi: 10.1038/ismej.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille R, Dingwall S, Wilcoxen J. The aerobic CO dehydrogenase from Oligotropha carboxidovorans. J Biol Inorg Chem. 2015;20:243–251. doi: 10.1007/s00775-014-1188-4. [DOI] [PubMed] [Google Scholar]
- Huerta-Cepas J, Forslund K, Coelho LP, et al. Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol Biol Evol. 2017;34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Nakamoto I, Omae K, et al. Structural and phylogenetic diversity of anaerobic carbon-monoxide dehydrogenases. Front Microbiol. 2019;9:3353. doi: 10.3389/fmicb.2018.03353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeoung J-H, Dobbek H. ATP-dependent substrate reduction at an [Fe8S9] double-cubane cluster. Proc Natl Acad Sci USA. 2018;115:2994–2999. doi: 10.1073/pnas.1720489115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, et al. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerby RL, Youn H, Roberts GP. RcoM: A new single-component transcriptional regulator of CO metabolism in bacteria. J Bacteriol. 2008;190:3336–3343. doi: 10.1128/JB.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori H, Inagaki S, Yoshioka S, et al. Crystal structure of CO-sensing transcription activator CooA bound to exogenous ligand imidazole. J Mol Biol. 2007;367:864–871. doi: 10.1016/j.jmb.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Li B, Elliott SJ. The catalytic bias of 2-oxoacid:ferredoxin oxidoreductase in CO2: evolution and reduction through a ferredoxin-mediated electrocatalytic assay. Electrochim Acta. 2016;199:349–356. doi: 10.1016/j.electacta.2016.02.119. [DOI] [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Strain-Damerell CM, Xie K, et al. Structural basis for NADH/NAD+ redox sensing by a rex family repressor. Mol Cell. 2010;38:563–575. doi: 10.1016/j.molcel.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Mohr T, Aliyu H, Küchlin R, et al. Comparative genomic analysis of Parageobacillus thermoglucosidasius strains with distinct hydrogenogenic capacities. BMC Genomics. 2018;19:880. doi: 10.1186/s12864-018-5302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y-J, Kwon J, Yun S-H, et al. Proteome analyses of hydrogen-producing hyperthermophilic archaeon Thermococcus onnurineus NA1 in different one-carbon substrate culture conditions. Mol Cell Proteomics. 2012;11(M111):015420. doi: 10.1074/mcp.m111.015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat CG, Chapman SK. Multi-heme cytochromes—new structures, new chemistry. Dalt Trans. 2005;2005:3381–3389. doi: 10.1039/b505184c. [DOI] [PubMed] [Google Scholar]
- NCBI Resource Coordinators Database resources of the national center for biotechnology information. Nucleic Acids Res. 2018;46:D8–D13. doi: 10.1093/nar/gkx1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger E, Rother M. Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch Microbiol. 2008;190:257–269. doi: 10.1007/s00203-008-0382-6. [DOI] [PubMed] [Google Scholar]
- Omae K, Yoneda Y, Fukuyama Y, et al. Genomic analysis of Calderihabitans maritimus KKC1, a thermophilic, hydrogenogenic, carboxydotrophic bacterium isolated from marine sediment. Appl Environ Microbiol. 2017;83:e00832-17. doi: 10.1128/AEM.00832-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N, Lippert KD. Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol. 1966;55:245–256. doi: 10.1007/BF00410246. [DOI] [Google Scholar]
- Poehlein A, Böer T, Steensen K, Daniel R. Draft genome sequence of the hydrogenogenic carboxydotroph Moorella stamsii DSM 26271. Genome Announc. 2018;6:e00345-18. doi: 10.1128/genomea.00345-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale SW. Life with carbon monoxide. Crit Rev Biochem Mol Biol. 2004;39:165–195. doi: 10.1080/10409230490496577. [DOI] [PubMed] [Google Scholar]
- Rajeev L, Hillesland KL, Zane GM, et al. Deletion of the Desulfovibrio vulgaris carbon monoxide sensor invokes global changes in transcription. J Bacteriol. 2012;194:5783–5793. doi: 10.1128/JB.00749-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravcheev DA, Li X, Latif H, et al. Transcriptional regulation of central carbon and energy metabolism in bacteria by redox-responsive repressor rex. J Bacteriol. 2012;194:1145–1157. doi: 10.1128/JB.06412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DJ, Sawers G, Van Spanning RJM. Periplasmic Electron Transport Systems in Bacteria. In: Lennarz WJ, Lane MD, editors. Encyclopedia of Biological Chemistry. New York: Elsevier; 2004. pp. 231–238. [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander K, Chung D, Hyatt D, et al. Rex in Caldicellulosiruptor bescii: Novel regulon members and its effect on the production of ethanol and overflow metabolites. Microbiologyopen. 2019;8:e00639. doi: 10.1002/mbo3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander K, Wilson CM, Rodriguez M, et al. Clostridium thermocellum DSM 1313 transcriptional responses to redox perturbation. Biotechnol Biofuels. 2015;8:211. doi: 10.1186/s13068-015-0394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AA, Venceslau SS, Grein F, et al. A protein trisulfide couples dissimilatory sulfate reduction to energy conservation. Science. 2015;350:1541–1545. doi: 10.1126/science.aad3558. [DOI] [PubMed] [Google Scholar]
- Schoelmerich MC, Müller V. Energy conservation by a hydrogenase-dependent chemiosmotic mechanism in an ancient metabolic pathway. Proc Natl Acad Sci USA. 2019;116:6329–6334. doi: 10.1073/pnas.1818580116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schut GJ, Lipscomb GL, Nguyen DMN, et al. Heterologous production of an energy-conserving carbon monoxide dehydrogenase complex in the hyperthermophile Pyrococcus furiosus. Front Microbiol. 2016;7:29. doi: 10.3389/fmicb.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelver D, Kerby RL, He Y, Roberts GP. Carbon monoxide-induced activation of gene expression in Rhodospirillum rubrum requires the product of cooA, a member of the cyclic AMP receptor protein family of transcriptional regulators. J Bacteriol. 1995;177:2157–2163. doi: 10.1128/jb.177.8.2157-2163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Song Y, Jeong Y, Cho BK. Analysis of the core genome and pan-genome of autotrophic acetogenic bacteria. Front Microbiol. 2016;7:1531. doi: 10.3389/fmicb.2016.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SW, Hirst MB, Ludden PW. CO-dependent H2 evolution by Rhodospirillum rubrum: role of CODH:CooF complex. Biochim Biophys Acta - Bioenerg. 2006;1757:1582–1591. doi: 10.1016/j.bbabio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Soboh B, Linder D, Hedderich R. Purification and catalytic properties of a CO-oxidizing:H2-evolving enzyme complex from Carboxydothermus hydrogenoformans. Eur J Biochem. 2002;269:5712–5721. doi: 10.1046/j.1432-1033.2002.03282.x. [DOI] [PubMed] [Google Scholar]
- Sokolova TG, Henstra AM, Sipma J, et al. Diversity and ecophysiological features of thermophilic carboxydotrophic anaerobes. FEMS Microbiol Ecol. 2009;68:131–141. doi: 10.1111/j.1574-6941.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- Søndergaard D, Pedersen CNS, Greening C. HydDB: A web tool for hydrogenase classification and analysis. Sci Rep. 2016;6:34212. doi: 10.1038/srep34212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels L, Krehenbrink M, Berks BC, et al. Thiosulfate reduction in Salmonella enterica is driven by the proton motive force. J Bacteriol. 2011;194:475–485. doi: 10.1128/JB.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlichny VA, Sokolova TG, Gerhardt M, et al. Carboxydothermus hydrogenoformans gen. nov., sp. nov., a CO-utilizing thermophilic anaerobic bacterium from hydrothermal environments of Kunashir Island. Syst Appl Microbiol. 1991;14:254–260. doi: 10.1016/S0723-2020(11)80377-2. [DOI] [Google Scholar]
- Techtmann SM, Colman AS, Robb FT. ‘That which does not kill us only makes us stronger’: the role of carbon monoxide in thermophilic microbial consortia. Environ Microbiol. 2009;11:1027–1037. doi: 10.1111/j.1462-2920.2009.01865.x. [DOI] [PubMed] [Google Scholar]
- Techtmann SM, Lebedinsky AV, Colman AS, et al. Evidence for horizontal gene transfer of anaerobic carbon monoxide dehydrogenases. Front Microbiol. 2012;3:132. doi: 10.3389/fmicb.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshchakov SV, Lebedinsky AV, Sokolova TG, et al. Genomic insights into energy metabolism of Carboxydocella thermautotrophica coupling hydrogenogenic CO oxidation with the reduction of Fe(III) minerals. Front Microbiol. 2018;9:1759. doi: 10.3389/fmicb.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoni MA, Curti B. Structure–function studies on the iron–sulfur flavoenzyme glutamate synthase: an unexpectedly complex self-regulated enzyme. Arch Biochem Biophys. 2005;433:193–211. doi: 10.1016/j.abb.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Venceslau SS, Stockdreher Y, Dahl C, Pereira IAC. The “bacterial heterodisulfide” DsrC is a key protein in dissimilatory sulfur metabolism. Biochim Biophys Acta - Bioenerg. 2014;1837:1148–1164. doi: 10.1016/j.bbabio.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Wolin EA, Wolin MJ, Wolfe RS. Formation of methane by bacterial extracts. J Biol Chem. 1963;238:2882–2886. [PubMed] [Google Scholar]
- Wu M, Ren Q, Durkin AS, et al. Life in hot carbon monoxide: the complete genome sequence of Carboxydothermus hydrogenoformans Z-2901. PLoS Genet. 2005;1:e65. doi: 10.1371/journal.pgen.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Ikeda T, Arai H, et al. Carboxylation reaction catalyzed by 2-oxoglutarate:ferredoxin oxidoreductases from Hydrogenobacter thermophilus. Extremophiles. 2010;14:79–85. doi: 10.1007/s00792-009-0289-4. [DOI] [PubMed] [Google Scholar]
- Yoneda Y, Yoshida T, Yasuda H, et al. A thermophilic, hydrogenogenic and carboxydotrophic bacterium, Calderihabitans maritimus gen. nov., sp. nov., from a marine sediment core of an undersea caldera. Int J Syst Evol Microbiol. 2013;63:3602–3608. doi: 10.1099/ijs.0.050468-0. [DOI] [PubMed] [Google Scholar]
- Zhang L, Nie X, Ravcheev DA, et al. Redox-responsive repressor rex modulates alcohol production and oxidative stress tolerance in Clostridium acetobutylicum. J Bacteriol. 2014;196:3949–3963. doi: 10.1128/JB.02037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Shi L. Genomic analyses of the quinol oxidases and/or quinone reductases involved in bacterial extracellular electron transfer. Front Microbiol. 2018;9:3029. doi: 10.3389/fmicb.2018.03029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw reads for RNA-Seq and the gene-expression profiles have been deposited in the NCBI/ENA/DDBJ Sequence Read Archive under accession number DRA008406.