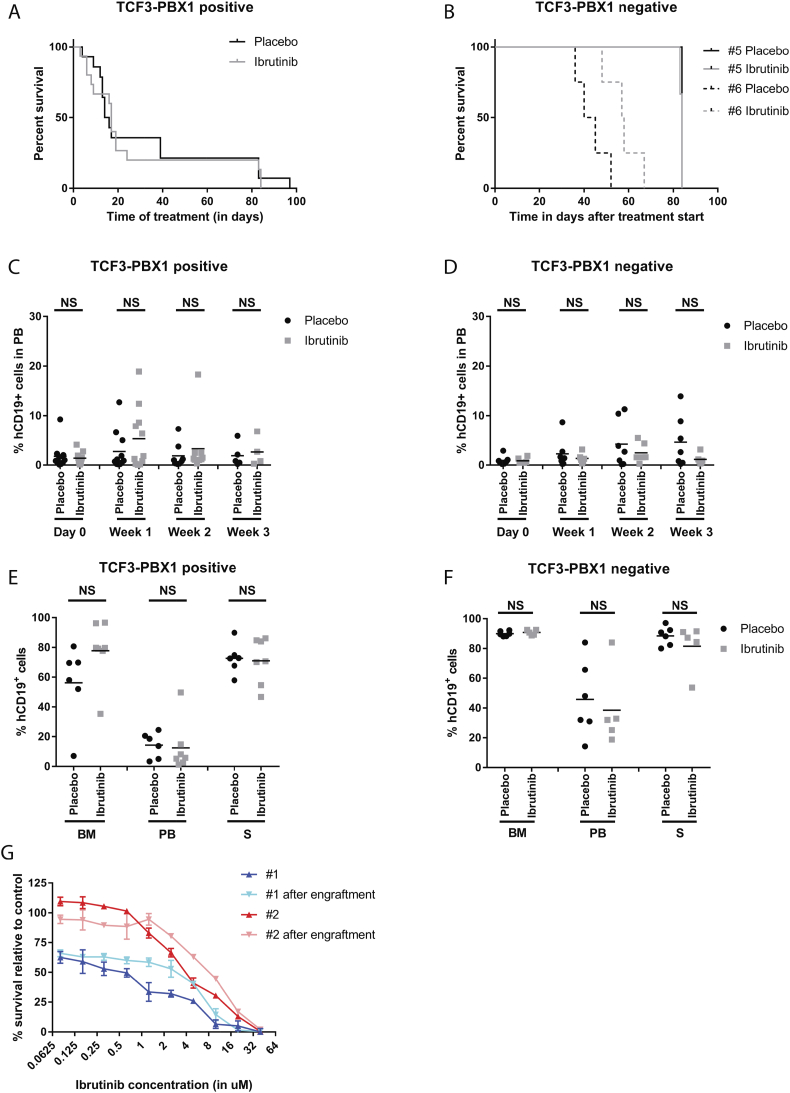
Fig. 2.
In vivo ibrutinib efficacy in xenografts of primary BCP-ALL samples. (A) Kaplan Meier curve for all TCF3-PBX1 positive engraftments treated with placebo (black line, n = 11) and 12 mg/kg ibrutinib (grey line, n = 11). (B) Kaplan Meier curve for TCF3-PBX1 negative cases BCP-ALL #5 (solid lines, n = 4 per treatment group) and BCP-ALL #6 (dotted lines, n = 4 per treatment group) engraftments treated with placebo (black lines) and 12 mg/kg ibrutinib (grey line). (C) Percentage of human CD19 positive cells (hCD19+) detected with flow cytometry in peripheral blood (PB) of in mice engrafted with TCF3-PBX1 positive cells at day 0 and 1, 2 and 3 weeks of treatment with ibrutinib (grey squares, n = 11 for day 0 and week 1, n = 9 for week 2, n = 4 for week 3) or placebo (black circles, n = 11 for day 0 and week 1, n = 8 for week 2, n = 5 for week 3). Treatment was started at day 1. Mean per treatment group is displayed. (D) Idem for TCF3-PBX1 negative cells, for ibrutinib (grey squares, n = 6 for all timepoints) or placebo (black circles, n = 7 for all timepoints). (E) Percentage of hCD19+ cells in bone marrow (BM), peripheral blood (PB) and spleen (S) of TCF3-PBX1 positive cells for placebo (black circles, n = 6) and ibrutinib (grey squares, n = 7). (F) Idem for TCF3-PBX1 negative cells, for placebo (black circles, n = 5) and ibrutinib (grey squares, n = 5). (G) Sensitivity of BCP-ALL # 1 (blue lines) and BCP-ALL #2 (red lines) to different concentrations of ibrutinib measured in MTT assays, for samples taken before injection into mice (#1: dark blue, #2 dark red) and after isolation from engrafted mice (#1: light blue, #2 light red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)