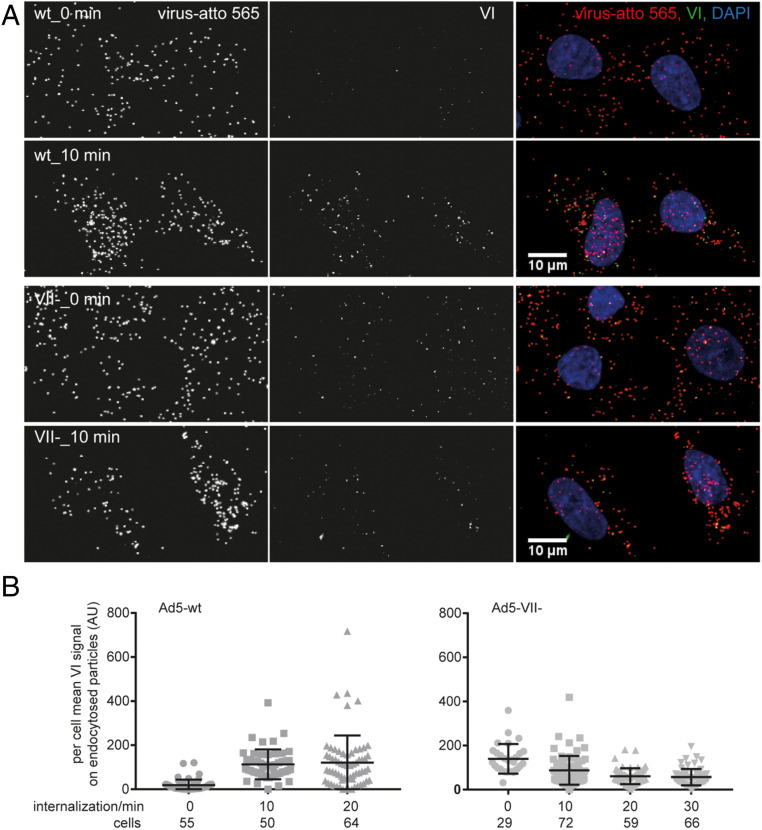
Fig. 1.
Reduced protein VI exposure in Ad5-VII- particles during entry in comparison to Ad5-wt. Atto 565-labeled virus particles were added to A549 cells at 4 °C for 60 min. Unbound particles were washed away, and cells were shifted to 37 °C for the indicated times. Intact cells were then incubated with mouse 9C12 antihexon antibody at 4 °C to tag plasma membrane-associated viruses, and after fixation, cells were permeabilized and stained with rabbit antiprotein VI antibody. These primary antibodies were detected with Alexa Fluor 680-conjugated anti-mouse and Alexa Fluor 488-conjugated anti-rabbit antibodies. Nuclei were stained with DAPI, and samples were imaged by confocal microscopy. (A) Representative images shown for Ad5-wt (Upper rows) and Ad5-VII- (Lower rows) at the 0-min and 10-min time points are maximum projections of confocal stacks. (Scale bars, 10 µm.) (B) The scatter plots show per cell mean protein VI signal on endocytosed particles (i.e., particles devoid of 9C12 antibody signal) for 10-min and 20-min time points, but the 0-min time point includes all cell-associated particles. One dot represents one cell and the number of cells analyzed is indicated. Error bars represent the means ± SD.