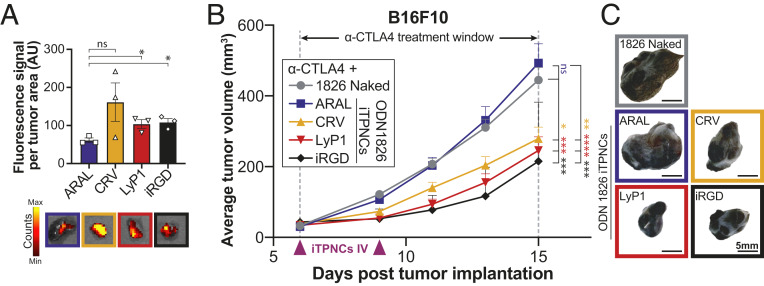
Fig. 4.
iTPNCs enhance responsiveness to anti-CTLA4 checkpoint inhibitor antibody therapy after systemic administration. (A) Fluorescence signal from iTPNCs synthesized with various homing peptides (CRV, LyP2, or iRGD) or nonhoming control peptide (ARAL) within B16F10 tumors after i.v. injection, measured by IVIS with excitation wavelength 535 nm and emission wavelength 580 nm. Bottom images show simultaneously captured fluorescent images overlaid on photos of representative tumors of each treatment group (*P < 0.05, unpaired t test). (B) Mice bearing B16F10 tumors were treated i.v. with ODN 1826 either naked or encapsulated in iTPNCs synthesized with homing peptides (CRV, LyP1, or iRGD) or nonhoming control peptide (ARAL), and were simultaneously treated intraperitoneally with 200 μg weekly of anti-CTLA4 (during the time window indicated). Treatments were initiated when tumors reached ∼30 mm3. Curves represent average tumor volume of B16F10 tumors from each treatment group: ODN 1826 naked (gray circles), ARAL-iTPNCs (blue squares), CRV-iTPNCs (gold triangles), LyP1-iTPNCs (red triangles), or iRGD-iTPNCs (black diamonds). (ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, two-way ANOVA; n = 18 to 20 tumors per group, error bars ± SEM, data are representative of at least two independent experiments). (C) Images of representative tumors from each treatment group shown in B. (Scale bars, 5 mm.)