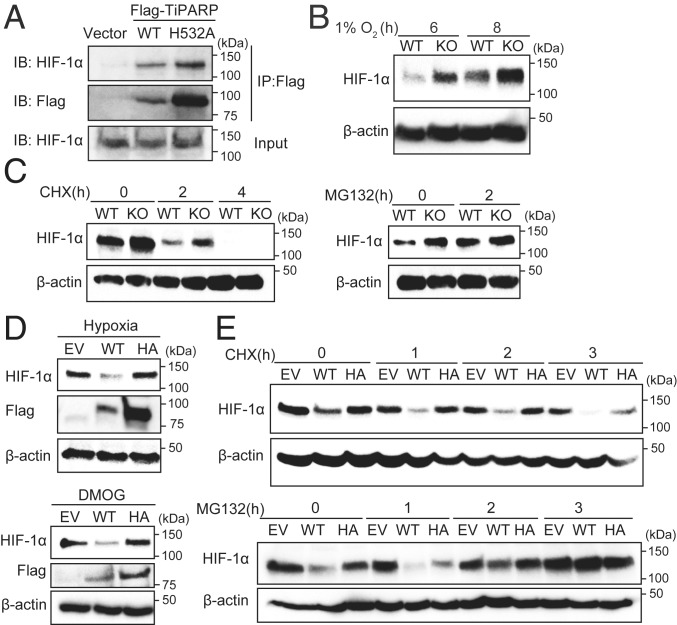
Fig. 2.
TiPARP promotes the degradation of HIF-1α in a catalytic activity-dependent manner. (A) HEK 293T cells were transfected with empty vector (EV), Flag-tagged WT TiPARP, or the H532A mutant. Cells were treated with 1 mM DMOG to stabilized endogenous HIF-1α protein. Co-IP of Flag-TiPARP and endogenous HIF-1α was detected by Western blot. Protein levels of WT TiPARP in cell lysates were very low due to its self-promoted degradation. (B) Western blot of HIF-1α in HAP-1 WT or TiPARP KO cells after 6 and 8 h of hypoxia (1% O2) treatment. (C) Western blot of HIF-1α in HAP-1 WT or TiPARP KO cells after 6 h of 1 mM DMOG treatment followed by treatment with 50 μM cycloheximide (CHX) (Left) or 20 μM MG132 (Right). (D) HCT116 cells were treated with 1 μg/mL doxycycline for 24 h to induce the expression of empty vector, Flag-tagged TiPARP WT, or inactive H532A mutant (HA). Cells were then incubated at hypoxia (1% O2) (Top) or with DMOG (Bottom) for 6 h. The protein level of HIF-1α was analyzed by Western blot. (E) Western blot of HIF-1α in HCT116 cells expressing empty vector, Flag-tagged WT TiPARP, or H532A mutant treated with DMOG for 6 h followed by treatment with CHX (Top) or MG132 (Bottom).