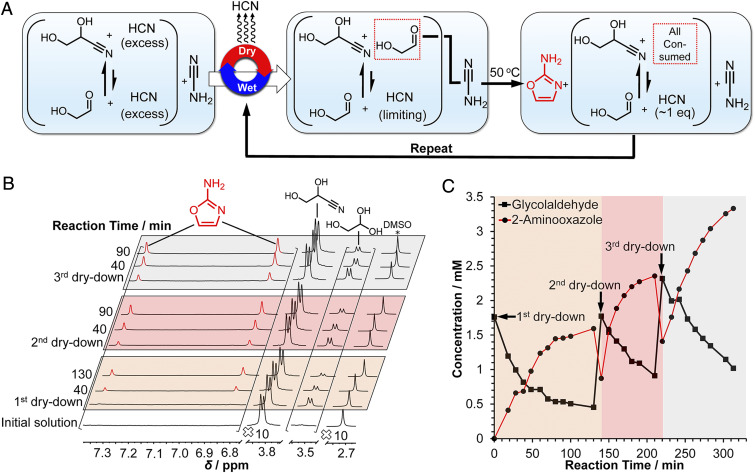
Fig. 1.
(A) Scheme for the synthesis of 2-aminooxazole from repeated dry-down cycles starting from a solution containing cyanamide, glyceronitrile, NaCN, and Pi. (B) NMR analysis of the dry-down reaction depicted in A. A 500-µL solution of 0.1 M cyanamide, 0.1 M glycolaldehyde, 0.25 M NaCN, and 0.5 M Pi was prepared in 10% D2O at pH 7. A volume of 0.5 µL of dimethyl sulfoxide (DMSO) was added as a standard, and the 1H NMR spectrum was recorded (initial solution). Next, the solution was dried down under reduced pressure at 30 °C and redissolved in 10% D2O. The mixture was then heated at 50 °C and monitored over time by 1H NMR spectroscopy. Two more dry–wet cycles were carried out in a similar manner for a total of three cycles. The intensities of the spectral regions containing 2-aminooxazole and the glycolaldehyde resonances were increased 10-fold for clarity. (C) A plot of the concentration versus time of glycolaldehyde and 2-aminooxazole determined by integration of the 1H NMR data shown in B against DMSO for the three dry–wet cycles.