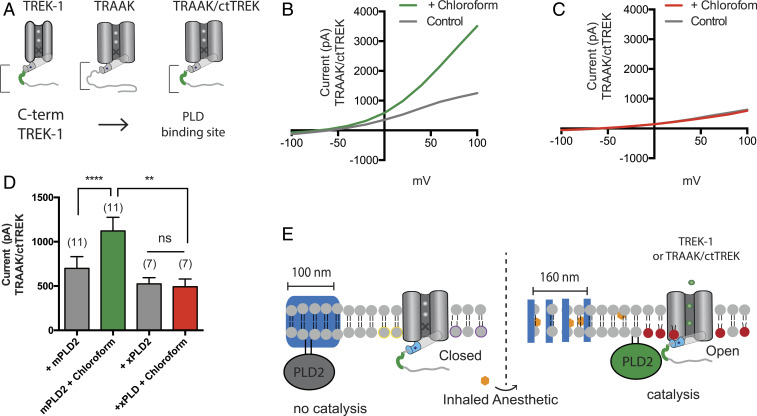
Fig. 3.
PLD2 localization renders TRAAK anesthetic sensitive. Native TRAAK is an anesthetic-insensitive channel. (A) Cartoon showing the experimental setup. TRAAK is fused with the C terminus of TREK-1 (TRAAK/ctTREK). The PLD2 binding site is depicted in green. (B and C) Representative I–V curve showing TRAAK/ctTREK-1 is activated by chloroform when coexpressed with mouse PLD2 (mPLD2) (B). The coexpression of the catalytically inactive PLD2 (xPLD2) abolishes the chloroform activation of TRAAK/ctTREK-1 chimeric channel (±SEM, n = 7) (C). (D) Bar graph summarizing TRAAK/ctTREK-1 chimeric channel current in the presence or absence of xPLD2 and chloroform (1 mM) at +40 mV (±SEM, n = 11) (Student’s t test results: ns P > 0.05; **P ≤ 0.01; ****P ≤ 0.0001). (E) Model mechanism showing that anesthetics activate the TRAAKctTREK-1 chimeric channel through raft disruption and PLD2 substrate presentation; xPLD2 abolishes the activation (the color scheme is as in Fig. 2).