FIGURE 3.
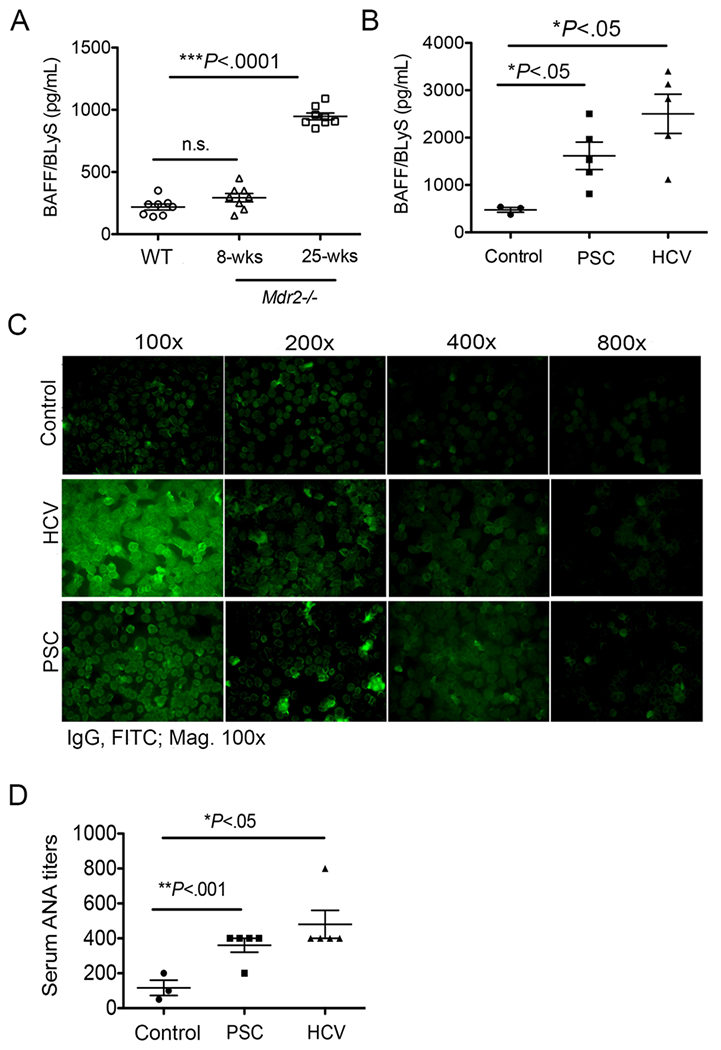
Elevated BAFF in Mdr2−/− mice and PSC human patients. (A) Serum specimens were collected from 8-weeks old and 25-weeks old Mdr2−/− and WT animals, and processed for detection of BAFF levels by using a standard mouse BAFF ELISA technique. Data are representative of at least 2 independent experiments (n = 3-5). (B) Plasma specimens from human subjects with end-stage liver disease (ESLD) as a result of PSC (n=5) or HCV (n=5) or non-HCV, non-alcoholic liver disease, non-nonalcoholic fatty liver liver-related liver resectioning (nonfibrotic control; n = 3) were processed for detection of BAFF levels by using a standard human BAFF ELISA method. (C) Representative ANA images in human patients by using human specific-IgG immunofluorescence staining of Hep-2 substrate are shown. (D) ANA titers are shown in control, PSC and HCV human patients. Statistical significance was determined by one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. *P<.05, **P<.001, ***P<.0001. Error bars reflect SEM.
