Figure 1.
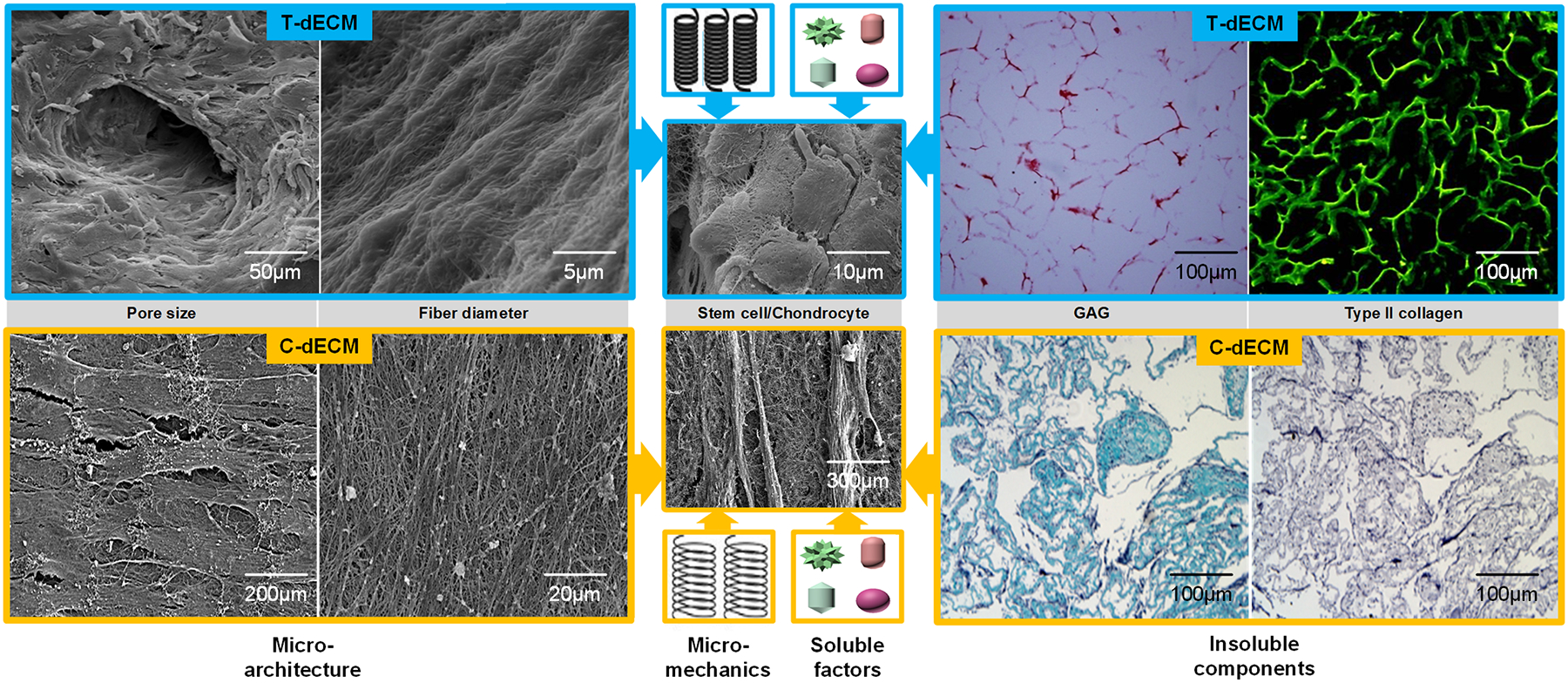
Diagram design. Decellularized extracellular matrix (dECM) from either tissue (T-dECM) or cell (C-dECM) supported chondrogenesis of stem cells and chondrocytes. Inherent properties and components of dECM scaffolds, such as microarchitecture characteristics including pore size and fiber diameter, micromechanical properties, insoluble components including glycosaminoglycan (GAG) and collagen, and soluble factors, were involved in the regulation of cell proliferation and chondrogenic differentiation. Cartilage T-dECMs with retained type II collagen and GAGs as well as larger pore sizes were more likely to facilitate seeded cells’ chondrogenic differentiation, which resulted in a round or elliptic morphology similar to chondrocyte-like cells [27]. C-dECMs with negligible levels of type II collagen and GAGs as well as smaller pore size had a superior tendency to support cell proliferation and chondrogenic potential, which led to a small and fibroblast-like shape [31]. Reprints with permission from “He, F.; Chen, X.; Pei, M. Tissue Eng. Part A 2009, 15, 3809. Copyright (2009) Mary Ann Liebert, Inc. Publications” and “Yang, Q., Peng, J., Guo, Q., Huang, J., Zhang, L., Yao, J., et al., Biomaterials 2008, 29, 2378. Copyright (2008) Elsevier Publications”.
