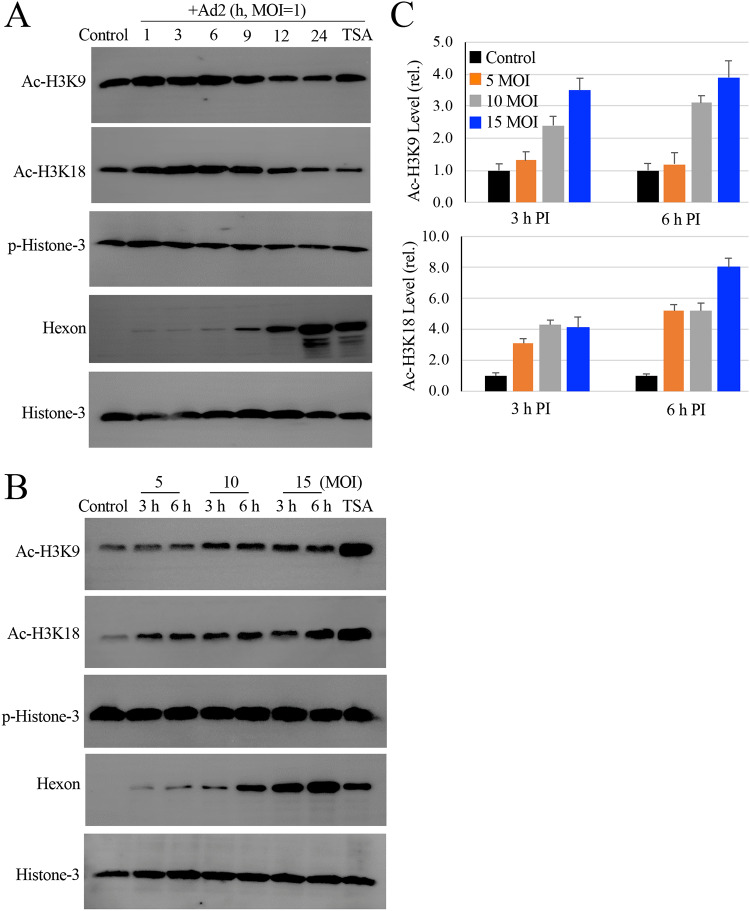
FIG 3.
Effect of HAdV infection on histone H3 modifications. (A) HAdV2 infection induces histone-3 acetylation. Monolayers of A549 cells were infected with HAdV2 at an MOI of 1 for times as indicated. The levels of acetylated and phosphorylated histome-3 (Ac-H3K9, Ac-H3K18, and p-H3 Ser 10) were determined by immunoblotting studies. Histone-3 expression and hexon production were also examined as loading and infection controls, respectively. TSA, Ad2 infected for 24 h in the presence of 150 nM TSA. The experiment was performed 2 times independently. (B and C) Histone-3 acetylation during HAdV2 infection. Monolayers of A549 cells were uninfected or infected with HAdV2 at 5 to 15 MOIs for 3 h or 6 h. TSA, Ad2 at 10 MOI for 6 h in the presence of 150 nM TSA. Histone-3 acetylation and phosphorylation was detected by immunoblotting assay using anti-Ac-H3K9, Ac-H3K18, or anti-p-Histone-3 (Ser10) antibody. (B) The expression of histone-3 and production of hexon was included as controls. (C) The relative intensities of Ac-H3K9 and anti-Ac-H3K18 were quantitatively measured and plotted.