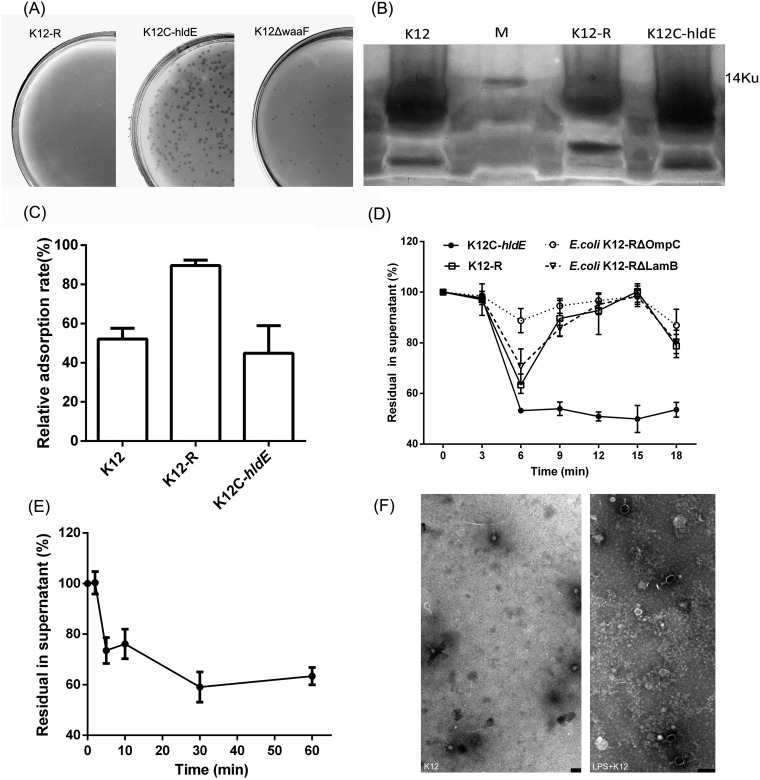
FIG 8.
LPS acted as the second receptor of phage Bp7. (A) The plasmid hldE-T was transferred into the mutant strain E. coli K-12-R to acquire the complement strain K-12C-hldE, the waaF gene of E. coli K-12 was knocked out by the no-SCAR system to construct the mutant strain E. coli K-12 ΔwaaF, and the susceptibility of E. coli K-12 ΔwaaF to phage Bp7 was tested by the plaque assay to identify the role of the hldE gene. Left, E. coli K-12-R was tolerant to phage Bp7; middle, the K-12C-hldE complemented strain carrying the hldE-T plasmid became susceptible to phage Bp7 after induced by IPTG; and right, the E. coli K-12 ΔwaaF was susceptible to phage Bp7. (B) LPS was purified from the E. coli K-12, E. coli K-12-R, and E. coli K-12C-hldE strains and detected by silver-staining SDS-PAGE. (C) Phage Bp7 was incubated with LPS of E. coli K-12, E. coli K-12-R, or E. coli K-12C-hldE, and free virions in the supernatant were tested by the plaque assay to identify the LPS effects on Bp7 adsorption in vitro. The adsorption rate of phage Bp7 was set as 100%. All assays were performed in triplicate. (D) The mutant strains of E. coli K-12-R ΔLamB and E. coli K-12-R ΔOmpC were constructed by the no-SCAR system, and the adsorption kinetics of Bp7 to E. coli K-12-R, E. coli K-12-R ΔLamB, E. coli K-12-R ΔOmpC, and E. coli K-12-R/hldE were tested by the plaque assay to identify the second receptor role of LPS. The phage Bp7 adsorption rates are shown as residual PFU percentages in supernatant. All assays were performed in triplicate. (E) The adsorption effects of phage Bp7 preincubated with LPS at different times was tested by a plaque assay. Phage Bp7 without LPS treatment was used as a control, and the adsorption rate was set as 100%. All assays were performed in triplicate. (F) LPS-triggered DNA release from phage Bp7 in vitro was tested by TEM. Phage Bp7 was used as a control (left); after incubation with LPS, phage Bp7 released its DNA, and the head of phage particles changed from white to black (right). Scale bar = 200 nm.