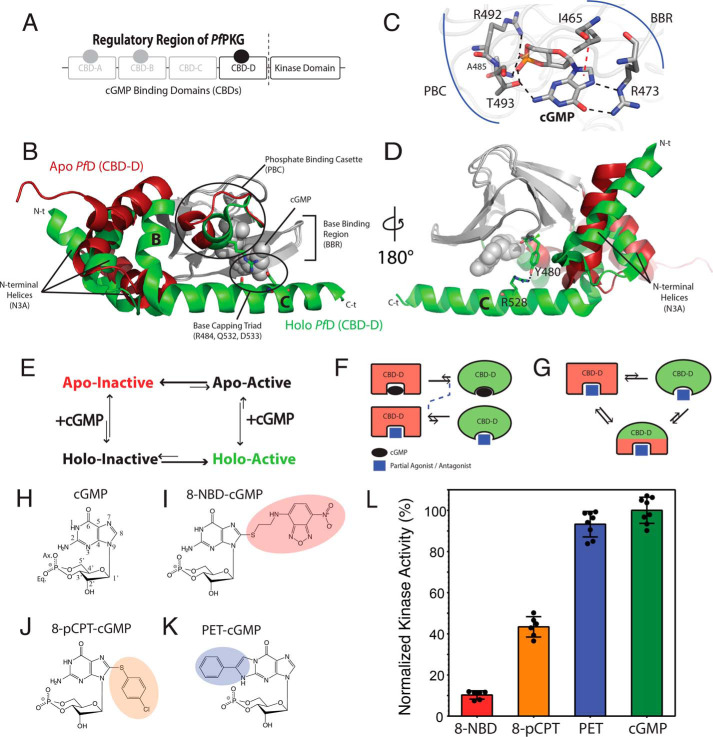
Figure 1.
Structural architecture of PfCBD-D and hypothetical models of inhibition. A, domain organization of PfPKG, including a regulatory region with four CBDs and a C-terminal catalytic kinase domain. CBD-C is degenerate and does not bind cGMP. B, structures of apo (red) and cGMP-bound (green) CBD-D (PfD). The invariant β-subdomain is gray in both structures. C, cGMP-binding pocket of PfD and selected cGMP-interacting residues. Black dashed lines, hydrogen bonds. Red dashed line, hydrophobic interaction. D, similar to B but with 180º vertical rotation, highlighting the Tyr480–Arg528 interaction. E, four-state thermodynamic equilibrium of the essential regulatory CBD (i.e. PfD). The apo PfD samples an autoinhibitory equilibrium between inactive and active states, which is coupled to the cGMP-binding equilibrium. F and G, hypotheses to explain PfPKG inhibition, as reversal of a two-state equilibrium (F) or stabilization of an intermediate with mixed active and inactive features and diminished activation competency (G). H–K, base-substituted cGMP analogs investigated here. L, maximum PfPKG kinase activities induced by cGMP and the cGMP analogs in I–K. Error bars represent the S.D.