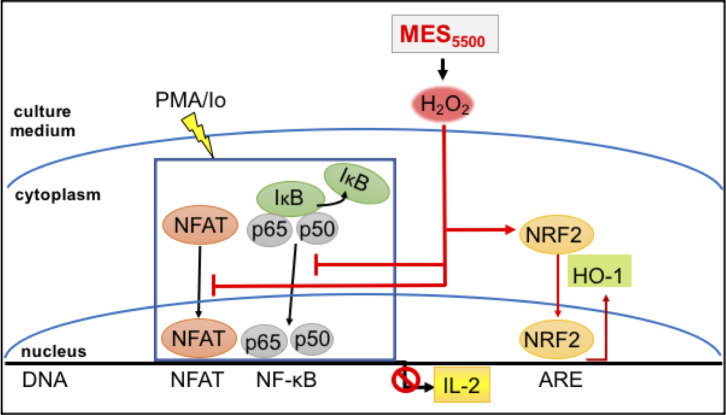
Fig 7. The schematic diagram of MES5500 mechanism.
Therapeutic use of electric current has been practiced for various kinds of inflammation, and the mechanism was thought to be its analgesic effect or by blood flow improvement [38]. It is well known that in an electrolytic reaction, electrical current generates H2O2 from water [39], and as shown in our study, this reaction might also occur in electrical stimulation used for therapeutic purposes. While many reports indicate that reactive oxygen species (ROS), such as H2O2, exerts pro-inflammatory effects through activation of NF-κB, more recent studies have shown that ROS are not only responsible for inducing inflammation, but also have potent anti-inflammatory properties [40, 41]. Increase in intracellular concentration of H2O2 have been demonstrated to diminish TLR4-induced activation of NF-κB and production of pro-inflammatory cytokines in neutrophils, epithelial cells and other cell populations [42]. Aside from T cells and primary splenocytes, MES5500 was also able to reduce the inflammatory cytokine expression in other cells such as MG6 (microglia), SHSY5Y (neuroblastoma lineage) (S5 Fig) and HeLa cells (S7 Fig). MES5500 did not have any effect at the basal state of these cells, indicating the safety of the treatment, and it modulated the excess reaction after inflammatory reagent stimulation. Moreover, MES5500 reduced the induction of IL-1β upon stimulation of these cells with lipopolysaccharide (LPS), an immune activator that binds to Toll-like receptor (TLR) 4 (S5A–S5C Fig). These data consolidate our findings that MES5500 has immune suppressor effect.