Abstract
The coronavirus disease 2019 (COVID-19) pandemic that hit the world in 2020 triggered a massive dissemination of information (an “infodemic”) about the disease that was channeled through the print, broadcast, web, and social media. This infodemic also included sensational and distorted information about drugs that likely first influenced opinion leaders and people particularly active on social media and then other people, thus affecting choices by individual patients everywhere. In particular, information has spread about some drugs approved for other indications (chloroquine, hydroxychloroquine, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, favipiravir, and umifenovir) that could have led to inappropriate and therefore hazardous use. In this article, we analyze the rationale behind the claims for use of these drugs in COVID-19, the communication about their effects on the disease, the consequences of this communication on people’s behavior, and the responses of some influential regulatory authorities in an attempt to minimize the actual or potential risks arising from this behavior. Finally, we discuss the role of pharmacovigilance stakeholders in emergency management and possible strategies to deal with other similar crises in the future.
Key Points
| The “infodemic,” or massive spread of information about the coronavirus 2019 (COVID-19) pandemic, often sensational and distorted, represented a novel challenge for health authorities. |
| This infodemic likely first influenced opinion leaders and people particularly active on social media and could have led to hazardous choices about the use of drugs. |
| In this scenario, pharmacovigilance must face different challenges, such as promoting clinical and observational studies, implementing spontaneous adverse drug reaction reporting systems and signal detection, and implementing and supporting risk communication strategies. |
Introduction
The coronavirus 2019 (COVID-19) pandemic triggered a domino effect that challenged all activities from the simplest (e.g., supermarket shopping) to the more complex (e.g., macro-economy) [1]. The pandemic also saw the parallel development of what has been called an “infodemic”, or a massive dissemination of information related to the disease through all media [2]. A substantial portion of this information concerned drugs that, although approved for other indications, could have potential efficacy in the prevention or treatment of COVID-19 [3]. Other commonly used drugs that could instead favor infection or an unfavorable prognosis were also subjects of media claims [4]. This information was often communicated in an inadequate, sensational, or distorted manner and was often supported by weak scientific evidence and may have influenced the behavior of many people in several countries, particularly frequent users of social media, with attendant potential risks related to the hazardous use of these drugs. Actual or potential events related to this behavior largely fall into the category of therapeutic errors [5] and have required pharmacovigilance stakeholders to activate risk minimization strategies.
In this article, we present some examples of drugs approved for other indications that have been the subject of media attention during the pandemic and for which misuse could lead to adverse events or drug-related problems. Then, we describe the way in which the communication took place and, finally, we discuss the role of pharmacovigilance stakeholders in managing this type of emergency.
Chloroquine and Hydroxychloroquine
In 2003, chloroquine was first used for the treatment of severe acute respiratory syndrome coronavirus (SARS-CoV). In particular, the combination of chloroquine and antiviral drugs (i.e., lopinavir and ritonavir) showed benefits for the treatment of SARS [6]. Savarino et al. [7] first highlighted the potential antiviral effects of chloroquine, a drug previously used in the treatment of malaria and rheumatic disorders. Indeed, chloroquine inhibits the pH-dependent steps of viral replication and exerts immunomodulatory effects. These mechanisms were confirmed by in vitro [8] and in vivo [9] experiments on SARS-CoV. After the COVID-19 outbreak, given the high genetic similarity between SARS-CoV-2 and SARS-CoV, chloroquine and hydroxychloroquine were immediately appealing for investigation in the treatment of COVID-19 [10]. A first clinical trial in patients with COVID-19 was reported on 12 February 2020 in the Chinese Clinical Trial Registry [11]. On 7 May 2020, there were 107 trials reported in the Clinicaltrials.gov repository.
The media claims surrounding these drugs, which were called “gamechangers”, enforced the illusion for many people that they could not only heal but also prevent the infection [12]. This illusion was corroborated by some governments when they rode the wave of enthusiasm for the results of a small open-label French study that had important methodological limitations [13]. Treatment with hydroxychloroquine was sometimes presented as being 100% effective, when the level of success was likely quite different [14, 15].
This storm of information had a detrimental impact, first on supply chain and availability and then on chloroquine and hydroxychloroquine utilization patterns. Indeed, several countries hastened to stockpile these drugs, even stopping their export [16], whereas the public started frantic purchasing of these drugs without prescription to ensure the availability of an emergency stock at home, thus reducing the supply for patients receiving chloroquine or hydroxychloroquine for rheumatic diseases [17, 18]. Thus, healthy people started taking uncontrolled doses of these drugs, mistakenly believing they could prevent COVID-19, which resulted in poisoning cases [16]. For instance, a fish tank cleaner product containing chloroquine phosphate caused the death of a man who self-administered the product in an attempt to prevent the infection [19]. Several serious adverse drug reactions (ADRs), such as sudden cardiac death due to arrhythmia and QT prolongation (especially in combination with azithromycin), have been reported for chloroquine, even in doses normally used to treat patients with COVID-19. Given the extent of these ADRs and the doubtful benefits, several hospitals in Sweden stopped using these drugs in patients with COVID-19 [20]. Recently, Mehra et al. [21] published the results of the first large multinational observational study to analyze registry data from 671 hospitals in six continents, including 96,032 patients hospitalized with COVID-19. They investigated the mortality associated with hydroxychloroquine exposure as a single therapeutic option or in combination with a macrolide and reported not only no benefit in patients with COVID-19 but also safety concerns, including decreased hospital survival and increased ventricular arrhythmias [21]. Of note, this study was a matter of debate in the scientific community because of possible methodological limitations and problems with sharing original data that may have affected the results [22]. Eventually, this debate led to the article being retracted [23].
To address this crisis, the regulatory health authorities that took action moved in two directions. First, clinical trials were encouraged at the beginning of the pandemic to verify the benefits and safety of potentially effective treatments [24–27], and second, adequate communication strategies were implemented with the issuance of safety alerts [26, 28–30] and clarification notes [31]. Notably, the World Health Organization (WHO) suspended clinical trials on the antimalarial drugs because of the emerging safety issues [32] reported by Mehra et al. [21], and the Italian Drug Agency stopped the use of hydroxychloroquine in patients with COVID-19 [33]. After the article was retracted [23], the WHO resumed investigations into the use of these drugs [34].
Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers
Drugs that act on the renin-angiotensin system (RAS), namely angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), likely triggered the greatest debate for their possible role in the modulation of COVID-19. This was because of the pivotal role of the ACE2 protein in both SARS-CoV-2 entry and the modulation of cytokines responsible for inflammation in acute respiratory distress syndrome. Indeed, spike proteins from coronaviruses, including SARS-CoV and SARS-CoV-2, interact with the ACE2 protein to enter human cells [35]. Since ACEIs and ARBs can induce upregulation of the ACE2 enzyme [36], it was postulated that an enhanced endogenous availability of ACE2 induced by antihypertensive drugs might favor virus entry into the host cell [37]. The observation of a high prevalence (> 70%) of hypertension in patients with COVID-19 [38, 39] could support this hypothesis. However, ACE2, through the ACE2/Ang (1–7)/Mas pathway, is a negative modulator of the RAS system, which in turn inhibits the release of several inflammatory cytokines. Therefore, any upregulation triggered by ACEIs and ARBs could improve the prognosis of COVID-19-related acute respiratory distress syndrome [40–42]. In this light, drugs acting on the RAS could exert beneficial or detrimental effects over different stages of the disease course. The discussion surrounding the central role of ACE2 in COVID-19 and the potential modulatory effect of drugs targeting this enzyme started with a letter by Fang et al. [37] to The Lancet Respiratory Medicine. This letter reviewed three sets of cases reported in China, including approximately 1300 patients with COVID-19, and highlighted an important percentage of patients with hypertension and diabetes (12–30%), and that such a frequency could have been explained by a high expression of ACE2 [37].
The difficult debate on the outcomes of ACE2 modulation by several drugs remained confined to the scientific community until the letter began to circulate online. It started with comments by some online newspapers and then spread through users of social networks and cross-platform messaging services [43, 44].
ACEIs and ARBs play a fundamental role in the prophylaxis and treatment of several cardiovascular diseases, and many patients worldwide were receiving treatment with these drugs. The uncontrolled diffusion of news about a presumed role of these drugs in favoring SARS-CoV-2 infection could have affected treatment compliance in many patients with serious implications for their health. However, there are currently no data to support this hypothesis, which therefore remains theoretical in nature.
Since information on the risk of COVID-19 to users of antihypertensive drugs was incomplete and because only continuously updated scientific evidence could support their appropriate use, some health authorities and scientific institutions rushed to rebut this news and recommend that therapies with antihypertensive drugs not be modified until the action is supported by the findings of ongoing studies [45–48]. In the meantime, observational studies assessing the outcomes of COVID-19 in patients using ACEIs or ARBs commenced [49]. Moreover, a multinational clinical trial is planned, where patients with COVID-19 taking ACEIs or ARB drugs will be randomized to switch to other antihypertensive drugs not targeting the RAS to investigate the effect of such discontinuation on disease outcomes [50]. The first results of some studies showed that these drugs have no influence on the prognosis of patients with COVID-19 [51–53]. Among these, a large population-based case–control study in the Lombardy region of Italy showed that neither ARBs nor ACEIs have a significant association with the risk of COVID-19 [53].
Non-Steroidal Anti-Inflammatory Drugs
The summaries of product characteristics for several nonsteroidal anti-inflammatory drugs (NSAIDs) contain warnings about the possibility that their pharmacological activity results in the reduction of inflammation and possibly of fever, thus masking and delaying the detection of symptoms of a worsening infection [54]. Of course, this warning includes COVID-19. Moreover, Fang et al. [37], in their abovementioned letter, noted NSAIDs, particularly ibuprofen, as being among those drugs that could increase ACE2 expression, thus potentially favoring SARS-CoV-2 infection. However, to the best of our knowledge, the only evidence supporting ibuprofen-associated ACE2 upregulation is a study on a model of cardiac fibrosis in rats [55].
On 14 March 2020, the French Health Minister tweeted a warning against the use of NSAIDs because they potentially aggravated COVID-19 infection [56]. This announcement was issued on the basis of reports from the French health authority about serious—but never published—adverse events associated with the use of NSAIDs in patients with COVID-19 [57]. In the same tweet, he recommended that acetaminophen should be taken in case of fever and that a physician or a pharmacist should be contacted for clarification [56]. This triggered an explosion of NSAID-related alerts posted or shared in social media and cross-platform messaging services, which were then amplified by articles in newspapers [58, 59].
Such widespread communication likely had an impact on a broad range of people and healthcare professionals and led to a decrease in the general use and prescription of ibuprofen (and other NSAIDs). On the other hand, acetaminophen consumption could have increased, with possible implications for the occurrence and reporting of related ADRs, including intoxication. However, to the best of our knowledge, these consequences remain speculative and should be investigated in future studies.
Several health authorities commented on the statements by French authorities. The WHO reported that no studies linked anti-inflammatory drugs with increased mortality rates from COVID-19 but added that experts were investigating to shed light on the issue. Nevertheless, the WHO did recommend the use of acetaminophen rather than ibuprofen in case of infection [60]. The European Medicines Agency (EMA) advised that there was no evidence to establish whether ibuprofen could aggravate the status of patients with COVID-19 and that patients and healthcare professionals must consider all accessible treatment alternatives, including acetaminophen and other NSAIDs, at the start of treatment for fever or pain in COVID-19 [61]. The EMA recommended that patients taking ibuprofen should not stop treatment, particularly those taking ibuprofen or other NSAIDs for chronic diseases. The agency also highlighted the need for prompt epidemiological studies to provide new and more specific evidence [61]. The US FDA, in line with statements issued by these regulatory agencies, remarked on the lack of evidence to support the warnings about ibuprofen [54]. Notably, an observational study has commenced in France to investigate the impact of ibuprofen and other medicines on the severity of COVID-19 [62].
Favipiravir
Favipiravir (Avigan®) is an RNA-dependent RNA polymerase inhibitor approved in Japan in 2014 for the treatment of influenza [63]. This drug has been used off-label to treat patients infected with Ebola virus and Lassa virus, but results have been uncertain [64]. The possibility of using favipiravir in the treatment of COVID-19 is poorly documented in the literature [65]. A study recently published in an engineering journal but with important methodological shortcomings reported that favipiravir had better efficacy in COVID-19 than did lopinavir/ritonavir [66], a combination whose effectiveness in COVID-19 has since been challenged [67].
On 22 March 2020, the director of the Chinese National Center for Biotechnology Development declared that favipiravir had “a high safety level and it’s clearly effective” in patients with COVID-19 [68]. This announcement was based on the results of a Chinese trial in 340 patients with COVID-19 showing that the virus tended to be cleared over 4 days in those who received the drug, versus 11 days in those who did not [68]. This news was published in the major European newspapers [68–70]. However, Fujifilm Toyama Chemical, the marketing authorization holder of favipiravir, stated that the drug was used only in an ongoing Japanese study conducted by medical institutions and distanced themselves from the Chinese study conducted using an equivalent favipiravir, citing insufficient clinical data to establish the efficacy and safety of Avigan® in patients with COVID-19 [71].
In Italy, support for the use of this drug was particularly amplified by a YouTube user who showed the streets of Tokyo without restrictions or social distancing, stating Japan had no infections because of the miracle drug favipiravir, a drug unavailable in Italy because of a presumed “government conspiracy” [72]. The video was shared on Facebook and Twitter many thousands of times, leading to high public expectations [69, 71].
Since the drug is unavailable in Europe and the USA, people began uncontrolled purchasing in both the exposed and the dark web [73], and Fujifilm Toyama Chemical declared they had received “a flood of inquiry from all over the world” [74]. Although there is currently no evidence of overdose or toxicity in people self-medicating with favipiravir in an attempt to treat or prevent COVID-19, this risk should be carefully considered by health authorities. In particular, mitochondrial toxicity cannot be excluded [75], and teratogenicity has been demonstrated [76]. Notably, a recent review of 29 studies of favipiravir confirmed a favorable safety profile [77]. An important concern could also be the selection of resistant and more aggressive virus strains in patients using favipiravir, as shown for the influenza virus [78, 79]. Safety issues led Japan’s Health, Labor and Welfare Ministry to limit the use of favipiravir to patients infected with novel or re-emerging influenza viruses resistant to other influenza antivirals [64].
On 31 March 2020, the president of Fujifilm Toyama Chemical announced the initiation of a phase III clinical trial to evaluate the safety and efficacy of Avigan® in patients with COVID-19 in Japan [80]. Meanwhile, the Italian Health Authority commented on the news circulated online and in newspapers to clarify the lack of evidence supporting the use of favipiravir in patients with COVID-19 [81, 82]. A clinical trial has been approved in Italy (NCT04336904), although at the time of writing it was not yet recruiting [83].
Umifenovir
Umifenovir (Arbidol®) is an antiviral developed by the Russian Research Chemical and Pharmaceutical Institute and approved in Russia and China for the prophylaxis and treatment of human influenza A and B infections and post-influenza complications. The mechanism of action is based predominantly on the impairment of critical steps in virus–cell interactions. For instance, in influenza virus, it interacts with hemagglutinin, causing an increase in hemagglutinin stability and thereby preventing the pH-induced transition of hemagglutinin into its functional fusogenic state [84]. Although umifenovir demonstrated a certain activity against respiratory viruses in in vitro [84] and in vivo [85] experiments, as well as in clinical trials [86] and observational studies [87, 88], no evidence exists to support its efficacy in the treatment or prevention of COVID-19.
Around the middle of March 2020, at Moscow airport, an Italian man posted a video in which he explained that umifenovir, a drug easily purchased in pharmacies, was the reason for the lower lethality of COVID-19 at that time in Russia compared with that in Italy [72]. Again, the video was spread on social networks and, within a few minutes, was shared 33,000 times [89]. This episode received media amplification in newspapers, particularly in Italy [90, 91].
The media claims surrounding umifenovir is thought to have caused uncontrolled purchasing of this drug on the internet [73, 92]. However, to the best of our knowledge, no umifenovir-related poisoning/toxicity has been reported with regards to attempts to treat or prevent COVID-19.
Nevertheless, the Italian health authority warned against the purchasing of drugs presumed to be effective against COVID-19 on unauthorized websites [93, 94]. Medical associations in Italy and Russia stated that data on efficacy were insufficient and the available clinical trials were not conclusive at all [95]. On 1 June 2020, nine studies aimed at investigating the effect of umifenovir in patients with COVID-19 were registered on clinicaltrials.gov [95].
Discussion
The COVID-19 outbreak has triggered several emergencies that spread in parallel with the viral epidemic. Among these, the uncontrolled diffusion of unreliable and unsubstantiated information concerning the disease and its treatment, for which the new word “infodemic” was coined [2], is one that health authorities are finding most difficult to fight. This infodemic covers many disease-related topics, spanning from debate about the origin (natural vs. artificial) and release (accidental vs. intentional) of SARS-CoV-2 through to vaccine opportunities [96, 97]. Drugs supposed to be helpful against the disease have become a matter of concern for all pharmacovigilance stakeholders. In this article, we have addressed the actual and potential impact of this infodemic on attitudes toward the correct use of drugs, particularly drugs that have emerged as potentially effective against the viral infection or involved in the management of the related disease.
The spreading of such an infodemic was likely favored by both the current hyper-interconnection of people (widespread use of smartphones, easy access to the internet, social media, and cross-platform messaging services) and populism [98]. Such terrain was particularly fertile for conspiracy theorists and profiteers. All media are involved in the spreading and amplification of information, and the communication process may have different patterns. Information can be generated in different ways, from the results of small or poorly conceived studies to reports highlighted in newspapers (such as with chloroquine, ibuprofen, or even ACEIs and ARBs), or may start in social media and “go viral” in a few hours (such as with antivirals). Among communication materials, short videos appear to be a particularly effective method of spreading information. Whatever the starting point, inadequate, sensational, or distorted information first affects opinion leaders, often politicians, and those active on social media, which, deliberate or not, lends consistency and reliability to the information. The final effect can be inadequate choices made by individuals everywhere.
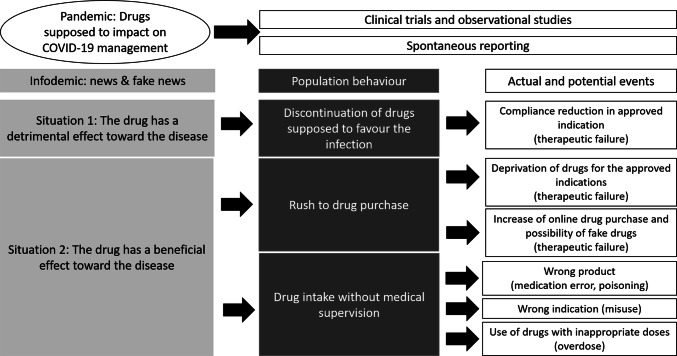
Two scenarios have been generated during the early phases of the pandemic (Fig. 1). In the first situation, drugs already approved for other indications have been presented as dangerous because of their ability to promote infection in healthy subjects or to lead to complications in patients with disease. The main consequence could be that patients who were taking these drugs for the approved indications could voluntarily discontinue therapy, risking a serious worsening of their underlying diseases. Whether this happened for both NSAIDs and ACEIs/ARBs should be verified in future studies.
Fig. 1.
“Infodemic” effects on people’s attitudes to developing uncritical behaviors around drug intake and possible consequences
In the second situation, some drugs that are already commercially available for other indications have been promoted as particularly effective despite a lack or complete absence of scientific evidence. The first effect on the behavior of many people was an initial rush to purchase the drug, which depleted stocks and then meant that patients using these drugs for approved indications were denied treatment. This was the case with chloroquine. Another consequence was the probable diffusion of falsified products, for which safety problems cannot be excluded in the face of a definite lack of efficacy. This has affected almost all drugs but particularly antivirals. For instance, although there is no evidence of a relationship with the pandemic, at the beginning of May 2020, authors from Africa reported the discovery of falsified chloroquine [99]. The second effect on the behavior of many people involved drugs being taken without medical supervision. This situation can have three types of consequences: (1) overdose, facilitated by the common belief that “more is better”; (2) intake independent from therapeutic rationale (these drugs are often promoted by describing a generic benefit, such as “the drug is beneficial against the virus”, and individuals cannot distinguish between preventive use in healthy people, post-exposure prophylaxis, and treatment of patients with disease); (3) intake of dangerous preparations with names or contents similar to those of promoted drugs, with a consequent risk of poisoning (the patient who died after self-administration of the fish tank cleaner containing chloroquine illustrates this final point [19]).
In this scenario, pharmacovigilance stakeholders are involved at various levels and face different challenges (Fig. 2). First, governments, health authorities, and manufacturers must collaborate to stimulate and support academia and research institutions in conducting studies to ensure that the risk–benefit profile of drug candidates for the treatment of COVID-19 is adequate. This includes not only clinical trials but also observational studies, particularly those of real-world evidence, which correlate the trends of drug use with clinical outcomes through evaluation of healthcare databases. In this regard, ethics committees have a key role to play in the proper evaluation of protocols and risk–benefit assessments, which must be tailored to the drugs and their indications (infection prevention, treatment of active disease, off-label use, compassionate use) and to the patients (critically or non-critically ill). Notably, several institutions were very efficient at developing COVID-19-specific guidelines in a few weeks, which were very helpful for caregivers involved in investigations of anti-COVID-19 drugs all over the world [100–102]. The collaboration of these investigators is also essential to ensure quality in data collection and avoid dispersion of information with detrimental effects on study quality [103]. Given the large number of articles being published on this topic in the scientific literature, health authorities should carefully consider the quality of evidence before taking decisions. As such, the retracted article about hydroxychloroquine safety issues [23] represents an important lesson.
Fig. 2.
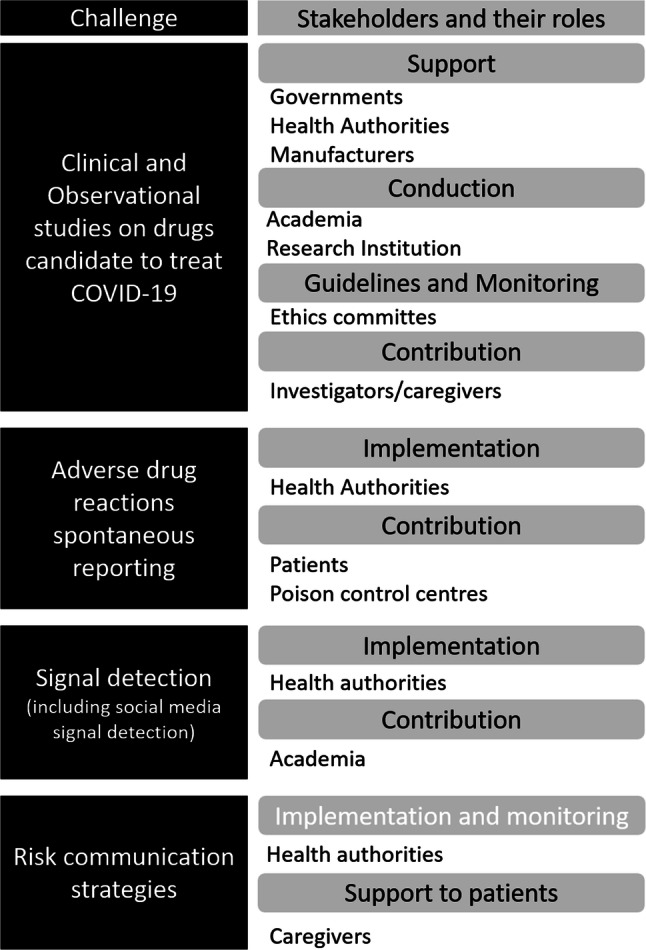
Challenges for pharmacovigilance activities, stakeholders and their roles
Another challenge is the implementation of spontaneous ADR reporting systems. In situations where the risk–benefit profile is unclear, it is important to monitor patient safety by stimulating spontaneous reporting of ADRs, particularly direct patient reports given the probability of issues related to self-medication, and frequently evaluating these reports. In this regard, strong support should come from poison control center networks and drug information services that may provide free, 24-h professional advice and medical management information to patients and healthcare professionals regarding exposure to medications. These centers may support with important information on the activity of regulatory agencies in the identification of drug misuse that may lead to accidental intoxication. For instance, in the first 3 months of 2020 in the USA, the National Poison Data System, Centers for Disease Control and Prevention, and the American Association of Poison Control Centers surveillance team detected a 20% increase in calls about exposures to cleaners and disinfectants compared with the same period in 2019 [104]. Although a direct link remains to be verified, it is reasonable to think that these calls were related to hygiene rules (not necessarily correct) that were spread widely by media.
Signal detection also deserves attention. We can expect the pandemic to have important effects on spontaneous reporting trends, both for drugs involved in the treatment of COVID-19 and for other drugs. Therefore, health authorities and academia will have to consider the “pandemic factor” in the interpretation of information obtained with the standard signal detection procedures in the future. One type of study that could be worth exploring is that of signal detection on the internet or social media. In standard signal detection procedures, this practice has shown limited utility in the face of substantial logistical work [105]. However, some authors have hypothesized its utility in evaluation by the public of risk perception related to drugs [106]. In the current scenario, considering the media engine that drives the behaviors related to inappropriate drug intake, it is possible that such studies (for example, sentiment analysis or dark web marketing analysis) could identify certain dangerous attitudes among drug users and support the development of adequate communication strategies to mitigate the related risk. They could also assess the effectiveness of these communication strategies.
Risk communication is the most difficult situation for pharmacovigilance stakeholders to manage. In recent months, all regulatory agencies have been involved in a fight against false or distorted information, intervening with clarifications and recommendations. Communication strategies, based on written or verbal recommendations and often supported by expert scientists, could have reduced effectiveness in a situation where functional illiteracy is widespread [107]. This is why fake news is very effective; it uses a lot of graphics and few words and the “click bait strategy” with a relevant empathic effect [108]. In our opinion, effective communication to support correct behavior should use empathy-based strategies in the same way [109], for example, by telling the stories of people who experienced significant consequences from the reckless use of drugs or employing the protagonists of these stories as testimonials. Certainly, healthcare professionals, who are the main holders of patient trust, must be involved in these campaigns to communicate correct drug use.
Conclusions
The pandemic has placed a strain on health systems, including pharmacovigilance systems, worldwide. The available systems were probably not ready to handle an emergency of such large proportions and with such unpredictable characteristics. The role of pharmacovigilance in the management of such emergencies is crucial, specifically the appropriate use of drugs proposed to treat the disease and the management of actual or potential consequences of inappropriate use of the same drugs. New challenges will probably arise once vaccines become available. It is important to learn from the mistakes made at this stage to stay ready to face the coming challenges.
Compliance with Ethical Standards
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
Marco Tuccori, Irma Convertino, Sara Ferraro, Emiliano Cappello, Giulia Valdiserra, Daniele Focosi, and Corrado Blandizzi have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors agree with the publication of this paper.
Availability of Data and Material (Data Transparency)
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Code Availability (Software Application or Custom Code)
Not applicable.
References
- 1.Siordia JA. Epidemiology and clinical features of COVID-19: a review of current literature. J Clin Virol [Internet]. 2020;127:104357. http://www.ncbi.nlm.nih.gov/pubmed/32305884. [DOI] [PMC free article] [PubMed]
- 2.Zarocostas J. How to fight an infodemic. Lancet (London, England). NLM (Medline); 2020;395:676. [DOI] [PMC free article] [PubMed]
- 3.Ahn D-G, Shin H-J, Kim M-H, Lee S, Kim H-S, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. Korea (South) 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 Infe. J Am Heart Assoc. England; 2020;9:e016219. [DOI] [PMC free article] [PubMed]
- 5.Aronson JK. Medication errors: what they are, how they happen, and how to avoid them. QJM. England. 2009;102:513–521. doi: 10.1093/qjmed/hcp052. [DOI] [PubMed] [Google Scholar]
- 6.Coronavirus: come si è arrivati alla sperimentazione della Clorochina—ISS [Internet]. [cited 2020 Apr 7]. https://www.iss.it/coronavirus/-/asset_publisher/1SRKHcCJJQ7E/content/id/5269460.
- 7.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect. Dis. Lancet Publishing Group; 2003. p. 722–7. [DOI] [PMC free article] [PubMed]
- 8.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keyaerts E, Li S, Vijgen L, Rysman E, Verbeeck J, Van Ranst M, et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. Elsevier BV; 2020;105938. [DOI] [PMC free article] [PubMed]
- 11.Shanping J. A prospective cohort study for the efficacy and safety of chloroquine in hospitalized patients with novel coronavirus pneumonia (COVID-19) (ChiCTR2000029542) [Internet]. Chinese Clin. Trial Regist. 2020 [cited 2020 Apr 27]. http://www.chictr.org.cn/showprojen.aspx?proj=48968.
- 12.Grady D, Thomas K. With Minimal Evidence, Trump Asks F.D.A. to Study Malaria Drugs for Coronavirus [Internet]. New York Times. [cited 2020 Apr 27]. https://www.nytimes.com/2020/03/19/health/coronavirus-drugs-chloroquine.html.
- 13.Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Netherlands; 2020;105949. [DOI] [PMC free article] [PubMed] [Retracted]
- 14.Wong, Julia C. Hydroxychloroquine: how an unproven drug became Trump’s coronavirus “miracle cure”. The Guardian. Guard [Internet]. https://www.theguardian.com/world/2020/apr/06/hydroxychloroquine-trump-coronavirus-drug.
- 15.Mahévas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. Bmj. 2020;2019:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaumont P, Ratcliffe R. Chloroquine: Trump’s misleading claims spark hoarding and overdoses [Internet]. Guard. [cited 2020 Apr 27]. https://www.theguardian.com/science/2020/mar/25/can-chloroquine-really-help-treat-coronavirus-patients.
- 17.Boseley S. Vital drug for people with lupus running out after unproven Covid-19 link [Internet]. Guard. 2020 [cited 2020 Apr 27]. https://www.theguardian.com/world/2020/mar/27/vital-drug-people-lupus-coronavirus-covid-19-link-hydroxychloroquine.
- 18.[no author listed]. COVID-19: nuove indicazioni AIFA per gestire il rischio di carenza di idrossiclorochina [Internet]. AIFA Off. website. [cited 2020 May 5]. https://www.aifa.gov.it/-/covid-19-nuove-indicazioni-aifa-per-gestire-il-rischio-di-carenza-di-idrossiclorochina.
- 19.Sheperd K. A man thought aquarium cleaner with the same name as the anti-viral drug chloroquine would prevent coronavirus. It killed him. [Internet]. Washington Post. [cited 2020 Apr 27]. https://www.washingtonpost.com/nation/2020/03/24/coronavirus-chloroquine-poisoning-death/.
- 20.McCall R. Some Swedish Hospitals have stopped using chloroquine to treat COVID-19 after reports of severe SIDE effects [Internet]. Newsweek. [cited 2020 Apr 27]. https://www.newsweek.com/swedish-hospitals-chloroquine-covid-19-side-effects-1496368.
- 21.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet (London, England) [Internet]. 2020;6736:31290. http://www.ncbi.nlm.nih.gov/pubmed/32450107. [DOI] [PMC free article] [PubMed] [Retracted]
- 22.O’Riordan M. Lancet COVID-19 Hydroxychloroquine Study Faces ‘Data Integrity’ Questions [Internet]. tctmd.com. 2020 [cited 2020 Jun 3]. https://www.tctmd.com/news/lancet-covid-19-hydroxychloroquine-study-faces-data-integrity-questions.
- 23.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet (London, England) [Internet]. Elsevier Ltd; 2020;6736:31324. http://www.ncbi.nlm.nih.gov/pubmed/32450107. [DOI] [PMC free article] [PubMed] [Retracted]
- 24.Kupferschmidt K, Cohen J. WHO launches global megatrial of the four most promising coronavirus treatments [Internet]. Science (80-.). [cited 2020 Apr 28]. https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-four-most-promising-coronavirus-treatments.
- 25.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;3–7. [DOI] [PMC free article] [PubMed]
- 26.[no author listed]. COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes [Internet]. Eurpean Med. Agency Off. Webpage. [cited 2020 Apr 28]. https://www.ema.europa.eu/en/news/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes.
- 27.[no author listed]. Fact sheet for patients and parents/caregivers emergency use authorization (EUA) of hydroxychloroquine sulfate for treatment of Covid-19 in certain hospitalized patients [Internet]. Food Drug Adm. Off. Webpage. [cited 2020 Apr 28]. https://www.fda.gov/media/136538/download.
- 28.Comunicazione AIFA sull’utilizzo di Clorochina e Idrossiclorochina nella terapia dei pazienti affetti da COVID-19—Informazioni di sicurezza [Internet]. [cited 2020 Apr 2]. https://www.aifa.gov.it/-/comunicazione-aifa-sull-utilizzo-di-clorochina-e-idrossiclorochina-nella-terapia-dei-pazienti-affetti-da-covid-19-informazioni-di-sicurezza.
- 29.[no author listed]. FDA Letter to Stakeholders: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19 in Humans [Internet]. Food Drug Adm. Off. Website. [cited 2020 Apr 28]. https://www.fda.gov/animal-veterinary/product-safety-information/fda-letter-stakeholders-do-not-use-chloroquine-phosphate-intended-fish-treatment-covid-19-humans.
- 30.[no author listed]. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems [Internet]. FDA Off. Website. [cited 2020 May 5]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or.
- 31.Cuppini L. Aifa: Clorochina non approvata per prevenire infezione da Sars-CoV-2 [Internet]. Corr. della Sera. [cited 2020 Apr 28]. https://www.corriere.it/salute/malattie_infettive/20_aprile_01/aifa-clorochina-non-approvata-prevenire-infezione-sars-cov-2-53179152-7370-11ea-bc49-338bb9c7b205.shtml.
- 32.[no author listed]. Q&A : Hydroxychloroquine and COVID-19 [Internet]. World Heal. Organ. 2020 [cited 2020 Jun 3]. https://www.who.int/news-room/q-a-detail/q-a-hydroxychloroquine-and-covid-19.
- 33.[no author listed]. COVID-19. Le motivazioni della decisione AIFA sull’uso di idrossiclorochina e clorochina [Internet]. AIFA Off. website. 2020 [cited 2020 Jun 8]. https://www.aifa.gov.it/-/covid-19-le-motivazioni-della-decisione-aifa-sull-uso-di-idrossiclorochina-e-clorochina.
- 34.Park A. WHO Resumes Study of Hydroxychloroquine for Treating COVID-19 [Internet]. Time. 2020 [cited 2020 Jun 8]. https://time.com/5847664/who-hydroxychloroquine-covid-19/.
- 35.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 37.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med [Internet]. Elsevier Ltd; 2020;8:e21. 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed]
- 38.Sun ML, Yang JM, Sun YP, Su GH. [Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi [Internet]. 2020 [cited 2020 Apr 21];43:219–22. https://www.ncbi.nlm.nih.gov/pubmed/32061198. [DOI] [PubMed]
- 39.Lippi G, Mattiuzzi C, Sanchis-Gomar F, Henry BM. Clinical and demographic characteristics of patients dying from COVID-19 in Italy versus China. J Med Virol. United States; 2020. [DOI] [PMC free article] [PubMed]
- 40.Cheng H, Wang Y, Wang G-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. Wiley; 2020. [DOI] [PMC free article] [PubMed]
- 41.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Focosi D, Tuccori M, Maggi F. ACE Inhibitors and AT1R Blockers for COVID-2019: Friends or Foes ? Preprints [Internet]. 2020. https://www.preprints.org/manuscript/202004.0151/v2.
- 43.Kekatos, Mary; Blanchard S. High blood pressure and diabetes medication taken by 13 million Americans could raise the risk of serious coronavirus symptoms, scientists say. Dly Mail [Internet]. https://www.dailymail.co.uk/health/article-8109453/High-blood-pressure-diabetes-drugs-raise-risk-coronavirus-symptoms.html.
- 44.Puente D. Ibuprofene e coronavirus. Il falso testo Whatsapp associato al chirurgo Pascale del Galeazzi di Milano. Parte la denuncia [Internet]. Open Online. https://www.open.online/2020/03/17/ibuprofene-e-coronavirus-il-falso-test-whatsapp-associato-al-chirurgo-pascale-del-galeazzi-di-milano-parte-la-denuncia/.
- 45.Società Italiana dell’Ipertensione Arteriosa. Farmaci antiipertensivi e rischio di COVID-19. Il comunicato della SIIA [Internet]. 2020. https://siia.it/notizie-siia/farmaci-antiipertensivi-e-rischio-di-covid-19-il-comunicato-della-siia/.
- 46.Società Italiana di Farmacologia. Documento Informativo della Società Italiana di Farmacologia—Uso di Ace-Inibitori/Sartani ed infezione da COVID-19 [Internet]. 2020. https://www.sifweb.org/documenti/document_2020-03-13_documento-informativo-della-societa-italiana-di-farmacologia-uso-di-ace-inibitori-sartani-ed-infezione-da-covid-19.
- 47.Euroepan Society of Cardiology. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers [Internet]. 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang.
- 48.European Medicines Agency. EMA advises continued use of medicines for hypertension, heart or kidney disease during COVID-19 pandemic [Internet]. 2020. https://www.ema.europa.eu/en/news/ema-advises-continued-use-medicines-hypertension-heart-kidney-disease-during-covid-19-pandemic.
- 49.Di Castelnuovo A. ACE inhibitors, angiotensin II type-I receptor blockers and severity of COVID-19 (CODIV-ACE) (NCT04318418) [Internet]. [cited 2020 Apr 23]. https://clinicaltrials.gov/ct2/show/NCT04318418.
- 50.Ludwig M. Stopping ACE-inhibitors in COVID-19 (ACEI-COVID)(NCT04353596) [Internet]. 2020 [cited 2020 Apr 23]. https://clinicaltrials.gov/ct2/show/NCT04353596.
- 51.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. Massachusetts Medical Society; 2020. [DOI] [PMC free article] [PubMed] [Retracted]
- 52.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin–angiotensin–aldosterone system inhibitors and risk of covid-19. N Engl J Med. Massachusetts Medical Society; 2020. [DOI] [PMC free article] [PubMed]
- 53.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of covid-19. N Engl J Med. Massachusetts Medical Society; 2020. [DOI] [PMC free article] [PubMed]
- 54.Food and Drug Administration. FDA advises patients on use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19 | FDA [Internet]. [cited 2020 Apr 2]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19.
- 55.Qiao W, Wang C, Chen B, Zhang F, Liu Y, Lu Q, et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiol. S. Karger AG. 2015;131:97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 56.Willsher K. Anti-inflammatories may aggravate Covid-19, France advises | World news | The Guardian [Internet]. 2020 [cited 2020 Apr 1]. https://www.theguardian.com/world/2020/mar/14/anti-inflammatory-drugs-may-aggravate-coronavirus-infection.
- 57.Day M. Covid-19: European drugs agency to review safety of ibuprofen. BMJ [Internet]. 2020;368:m1168. http://www.ncbi.nlm.nih.gov/pubmed/32205306. [DOI] [PubMed]
- 58.Kolata G. Is ibuprofen really risky for coronavirus patients? [Internet]. New York Times. [cited 2020 May 5]. https://www.nytimes.com/2020/03/17/health/coronavirus-ibuprofen.html.
- 59.Schraer R, Goodman J, Coleman A. Coronavirus and ibuprofen: Separating fact from fiction [Internet]. BBC News Website. [cited 2020 May 5]. https://www.bbc.com/news/51929628.
- 60.Health Agencies: No Evidence Ibuprofen Worsens Coronavirus—The New York Times.
- 61.European Medicines Agency. EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19 | European Medicines Agency. Eur Med Agency [Internet]. 2020;31:18–9. https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19.
- 62.[no author listed]. Role of Ibuprofen and Other Medicines on Severity of Coronavirus Disease 2019 (COVID-19) Infections: a Case-control Study (NCT04383899) [Internet]. clinicaltrials.gov. 2020 [cited 2020 Jun 3]. https://clinicaltrials.gov/ct2/show/NCT04383899.
- 63.Jordan PC, Stevens SK, Deval J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir Chem Chemother. 2018;26:1–29. doi: 10.1177/2040206618764483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 66.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;4–10. [DOI] [PMC free article] [PubMed]
- 67.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;1787–99. [DOI] [PMC free article] [PubMed]
- 68.McCurry J. Japanese flu drug “clearly effective” in treating coronavirus, says China [Internet]. Guard. 2020 [cited 2020 Apr 27]. https://www.theguardian.com/world/2020/mar/18/japanese-flu-drug-clearly-effective-in-treating-coronavirus-says-china.
- 69.D’Aria I. Coronavirus, la speranza dell’antivirale Avigan e il video su Facebook. I dubbi dell’Aifa e la sperimentazione in Veneto [Internet]. La Repubb. 2020 [cited 2020 Apr 27]. https://www.repubblica.it/salute/medicina-e-ricerca/2020/03/22/news/coronavirus_a_tokio_la_speranza_di_un_anti-virale_che_funziona_da_domani_sperimentazione_anche_in_italia-251983590/.
- 70.Fujifilm décolle à Tokyo, l’un de ses médicaments efficace contre le Covid-19 selon Pékin—Le Figaro [Internet]. [cited 2020 Apr 20]. https://www.lefigaro.fr/flash-eco/fujifilm-decolle-a-tokyo-l-un-de-ses-medicaments-efficace-contre-le-covid-19-selon-pekin-20200318.
- 71.D’Aria I. Covid-19: Speranza, “Aifa procede su sperimentazione Avigan” [Internet]. La Repubb. 2020 [cited 2020 Apr 27]. https://www.repubblica.it/salute/medicina-e-ricerca/2020/03/23/news/covid-19_speranza_aifa_procede_su_sperimentazione_avigan_-252090636/.
- 72.[no author listed]. La bufala del farmaco Arbidol [Internet]. Corr. della Sera. 2020 [cited 2020 Apr 27]. https://video.corriere.it/bufala-farmaco-arbidol/277e5b16-6c3b-11ea-8403-94d97cb6fb9f.
- 73.[no author listed]. SPAIN : 58% OF ILLEGAL CORONAVIRUS MEDICINE AND MASK SUPPLIES CHANNELED THROUGH SOCIAL MEDIA [Internet]. Int. Inst. Res. Against Counterfeit Med. Website. [cited 2020 May 5]. https://www.iracm.com/en/2020/04/spain-58-illegal-coronavirus-medicine-mask-supplies-channeled-social-media/.
- 74.Kuchler, Hanna; Inagaki, Kana; Neville S. The global hunt for a coronavirus drug [Internet]. Finacial Times. 2020 [cited 2020 Apr 27]. https://www.ft.com/content/91bd081e-6e7b-11ea-9bca-bf503995cd6f.
- 75.Jin Z, Tucker K, Lin X, Kao CC, Shaw K, Tan H, et al. Biochemical evaluation of the inhibition properties of favipiravir and 2=-C-methyl-cytidine triphosphates against human and mouse norovirus RNA polymerases. Antimicrob Agents Chemother. American Society for Microbiology; 2015;59:7504–16. [DOI] [PMC free article] [PubMed]
- 76.Nagata T, Lefor AK, Hasegawa M, Ishii M. Favipiravir: A New Medication for the Ebola Virus Disease Pandemic. Disaster Med Public Health Prep. Cambridge University Press; 2015;9:79–81. [DOI] [PubMed]
- 77.Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir—a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6:45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldhill DH, Te Velthuis AJW, Fletcher RA, Langat P, Zambon M, Lackenby A, et al. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci USA. 2018;115:11613–11618. doi: 10.1073/pnas.1811345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ormond L, Liu P, Matuszewski S, Renzette N, Bank C, Zeldovich K, et al. The combined effect of oseltamivir and favipiravir on influenza a virus evolution. Genome Biol Evol. 2017;9:1913–1924. doi: 10.1093/gbe/evx138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.[no author listed]. Fujifilm announces the start of a phase III clinical trial of influenza antiviral drug “Avigan Tablet” on COVID-19 and commits to increasing production [Internet]. Fujifilm Off. website. 2020 [cited 2020 Apr 27]. https://www.fujifilm.com/jp/en/news/hq/3211.
- 81.Favipiravir: aggiornamento della valutazione della CTS [Internet]. [cited 2020 Apr 20]. https://www.aifa.gov.it/-/favipiravir-aggiornamento-della-valutazione-della-cts.
- 82.AIFA precisa, uso favipiravir per COVID-19 non autorizzato in Europa e USA, scarse evidenze scientifiche sull’efficacia [Internet]. [cited 2020 Apr 20]. https://www.aifa.gov.it/-/aifa-precisa-uso-favipiravir-per-covid-19-non-autorizzato-in-europa-e-usa-scarse-evidenze-scientifiche-sull-efficacia.
- 83.Rizzardini G. Clinical Study To Evaluate The Performance And Safety Of Favipiravir in COVID-19—NCT04336904 [Internet]. clincialtrial.gov. 2020 [cited 2020 Apr 27]. https://clinicaltrials.gov/ct2/show/NCT04336904?term = favipiravir&cond = Covid-19&draw=4&rank=1.
- 84.Haviernik J, Štefánik M, Fojtíková M, Kali S, Tordo N, Rudolf I, et al. Arbidol (Umifenovir): A broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses. MDPI AG; 2018;10. [DOI] [PMC free article] [PubMed]
- 85.Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol. 2007;152:1447–1455. doi: 10.1007/s00705-007-0974-5. [DOI] [PubMed] [Google Scholar]
- 86.Pshenichnaya NY, Bulgakova VA, Lvov NI, Poromov AA, Selkova EP, Grekova AI, et al. Clinical efficacy of umifenovir in influenza and ARVI (study ARBITR) Ter Arkh. Consilium Medikum. 2019;91:56–63. doi: 10.26442/00403660.2019.03.000127. [DOI] [PubMed] [Google Scholar]
- 87.Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. W.B. Saunders Ltd; 2020. [DOI] [PMC free article] [PubMed]
- 88.Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. Elsevier B.V.; 2020. [DOI] [PMC free article] [PubMed]
- 89.Puente, David; Pill J. Il complottone del farmaco Arbidol (o Abidol) contro il coronavirus non venduto in Italia. Fate attenzione! [Internet]. Open Online. 2020 [cited 2020 Apr 27]. https://www.open.online/2020/03/17/il-complottone-del-farmaco-arbidol-o-abidol-contro-il-coronavirus-non-venduto-in-italia-fate-attenzione/.
- 90.[no author listed]. Coronavirus, la fake news sul farmaco russo. Burioni: Non serve a niente, basta con le bufale [Internet]. Messagg. [cited 2020 Apr 27]. https://www.ilmessaggero.it/salute/medicina/coronavirus_farmaco_russia_arbidol_fake_news_17_marzo_2020-5116727.html.
- 91.Corrado P. “Arbidol, il farmaco russo che salva dal Coronavirus”. Ma è una bufala [Internet]. Il Secolo d’Italia. 2020 [cited 2020 Apr 27]. https://www.secoloditalia.it/2020/03/arbidol-il-farmaco-russo-che-salva-dal-coronavirus-ma-e-una-bufala-video/.
- 92.Dunn W. How coronavirus is worsening the UK’s problem with unlicensed drugs [Internet]. NewStatesman website. [cited 2020 May 5]. https://www.newstatesman.com/science-tech/coronavirus/2020/03/what-arbidol-russian-coronavirus-cure-being-sold-ebay.
- 93.Listed] [no author. COVID-19: attenzione ai medicinali falsificati provenienti da siti web non registrati [Internet]. AIFA Off. website. 2020 [cited 2020 Apr 27]. https://www.aifa.gov.it/web/guest/-/covid-19-attenzione-ai-medicinali-falsificati-provenienti-da-siti-web-non-registrati.
- 94.Valvo L, Bartolomei M, Gaudiano MC, Sestili I, Manna L, Antoniella E, et al. Indicazioni relative ai rischi di acquisto online di farmaci per la prevenzione e terapia dell’infezione COVID-19 e alla diffusione sui social network di informazioni false sulle terapie [Internet]. 2020 [cited 2020 Apr 27]. https://www.epicentro.iss.it/coronavirus/pdf/rapporto-covid-19-15-2020.pdf.
- 95.Di Grazia S. Esiste un farmaco russo che cura la Covid-19? [Internet]. Fed. Ital. dei Medici Chir. e Odontoiatr. Off. Website. 2020 [cited 2020 Apr 27]. https://dottoremaeveroche.it/esiste-un-farmaco-russo-che-cura-la-covid-19/.
- 96.Caddy S. Developing a vaccine for covid-19. BMJ. England; 2020. p. m1790. [DOI] [PubMed]
- 97.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orso D, Federici N, Copetti R, Vetrugno L, Bove T. Infodemic and the spread of fake news in the COVID-19-era. Eur J Emerg Med. England; 2020. [DOI] [PMC free article] [PubMed]
- 99.Gnegel G, Hauk C, Neci R, Mutombo G, Nyaah F, Wistuba D, et al. Identification of Falsified Chloroquine Tablets in Africa at the Time of the COVID-19 Pandemic. Am J Trop Med Hyg [Internet]. 2020; http://www.ncbi.nlm.nih.gov/pubmed/32400349. [DOI] [PMC free article] [PubMed]
- 100.[no author listed]. DH-BIO Statement on human rights considerations relevant to the COVID-19 pandemic [Internet]. 2020. https://rm.coe.int/inf-2020-2-statement-covid19-e/16809e2785.
- 101.[no author listed]. Position of the European Network of Research Ethics Committees (EUREC) on the Responsibility of Research Ethics Committees during the COVID-19 Pandemic. 2020.
- 102.[no author listed]. Guidance on the Management of clinical trials during the COVID-19 (corona virus) pandemic. 2020.
- 103.Glasziou PP, Sanders S, Hoffmann T. Waste in covid-19 research. BMJ [Internet]. 2020;369:m1847. 10.1136/bmj.m1847. [DOI] [PubMed]
- 104.Chang A, Schnall AH, Law R, Bronstein AC, Marraffa JM, Spiller HA, et al. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19—National Poison Data System, United States, January 1, 2020–March 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:496–498. doi: 10.15585/mmwr.mm6916e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caster O, Dietrich J, Kürzinger ML, Lerch M, Maskell S, Norén GN, et al. Assessment of the Utility of Social Media for Broad-Ranging Statistical Signal Detection in Pharmacovigilance: Results from the WEB-RADR Project. Drug Saf [Internet]. Springer International Publishing; 2018;41:1355–69. 10.1007/s40264-018-0699-2. [DOI] [PMC free article] [PubMed]
- 106.Convertino I, Ferraro S, Blandizzi C, Tuccori M. The usefulness of listening social media for pharmacovigilance purposes: a systematic review. Expert Opin Drug Saf [Internet]. Taylor and Francis Ltd; 2018 [cited 2020 Apr 1];17:1081–93. http://www.ncbi.nlm.nih.gov/pubmed/30285501. [DOI] [PubMed]
- 107.Vágvölgyi R, Coldea A, Dresler T, Schrader J, Nuerk HC. A review about functional illiteracy: Definition, cognitive, linguistic, and numerical aspects. Front Psychol. 2016;7:1–13. doi: 10.3389/fpsyg.2016.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pierri F, Piccardi C, Ceri S. Topology comparison of Twitter diffusion networks effectively reveals misleading information. Sci Rep [Internet]. Springer US; 2020;10:1–9. 10.1038/s41598-020-58166-5. [DOI] [PMC free article] [PubMed]
- 109.Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27:237–251. doi: 10.1177/0163278704267037. [DOI] [PubMed] [Google Scholar]