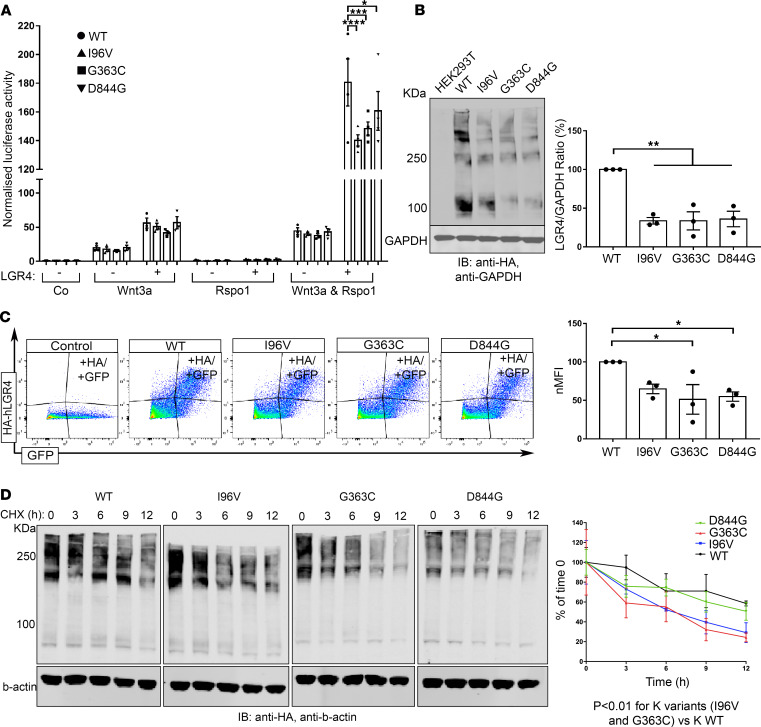
Figure 3. LGR4 mutant receptors affect Wnt/β-catenin signaling owing to defects in protein production, trafficking, and protein turnover.
(A) HEK293T were nontransfected (–) or transfected (+) with HA-hLGR4 plasmids (WT or mutants; 200 ng/well) and reporter vectors (TOP-Flash and Renilla [150 ng/well]). Signaling was activated with conditioned media treatment, and each transfection normalized by cotransfection with Renilla. HA-hLGR4 mutant receptors resulted in significant reduced luciferase activity. ****P < 0.0001, ***P = 0.004, *P = 0.0437; n = 4. (B) Western blot and densitometry analysis revealed reduced levels of LGR4 mutants. GAPDH was used as loading control. Molecular weight (KDa) of a protein standard is reported (left panel: WT vs. I96V **P = 0.0054; WT vs. G363C **P = 0.0039; WT vs. D844G **P = 0.0049); n = 3. (C) Representative plots and quantification of flow cytometry analysis of cell surface expression of WT and mutant LGR4 proteins expressed in HEK293T. Normalized median fluorescence intensity (nMFI) reveals reduced levels of mutant receptors at the plasma membrane compared with HA-hLGR4 WT (WT vs. p.G363C *P = 0.0228; WT vs. p.D844G *P = 0.0498). Control: HEK293T transfected with pcDNA3.1EGFP vector only. n = 3. (D) LGR4 mutants have a shorter half-life: transiently transfected HEK293T with HA-hLGR4 WT or mutant constructs treated with CHX (50 μg/mL) for different time periods (0, 3, 6, 9, and 12). Levels of LGR4 WT and mutant proteins were expressed relative to untreated LGR4 WT or mutant proteins (0 h); n = 4. Statistical analysis by 1-way ANOVA. Half-life was analyzed via a 1-phase decay equation, and degradation speed (K) compared between each mutant and WT protein using the extra sum-of-squares F test. Co, control medium; Rspo1, Rspondin-1.