Abstract
Low-density lipoprotein apheresis (LDL-A) has been developed as a therapy for familial hypercholesterolemia, but LDL-A has also been used as a general treatment for drug-resistant nephrotic syndrome (NS) due to focal segmental glomerulosclerosis (FSGS). The patients with NS due to minimal change disease (MCD) are often difficult to control effective circulating plasma volume, causes acute kidney injury (AKI), and when diuretics are not effective and the respiratory condition of patients worsens, patients require acute renal replacement therapy (ARRT). The effectiveness of LDL-A is not only reduction of serum low-density lipoprotein but also various other benefits. LDL-A might have improved renal hemodynamics by reducing vasoconstrictive eicosanoids and contributed to the therapeutic effect of antiproteinuric drugs such as corticosteroids. We treated a 49-year-old Japanese woman and a 71-year-old Japanese man with AKI caused by NS due to MCD, who required ARRT. Although these patients received ARRT and corticosteroids, their AKI and MCD did not improve sufficiently. We initiated LDL-A treatment for these patients as an additional treatment modality, because their total serum cholesterol levels were high at the time of admission. After the additional LDL-A treatment, both patients were able to discontinue ARRT, because NS and AKI in both patients were improved sufficiently. It is possible that early additional LDL-A is effective for patients with AKI and NS due to MCD who require ARRT, and may help patients discontinue ARRT because of the effect of LDL-A such as improving hypercoagulability and renal hemodynamics and contributing to the therapeutic effect of corticosteroids.
Keywords: low-density lipoprotein apheresis, minimal change disease, nephrotic syndrome, acute kidney injury, acute renal replacement therapy
Introduction
Low-density lipoprotein apheresis (LDL-A) has been developed as a therapy for familial hypercholesterolemia, but LDL-A has also been used as a general treatment for drug-resistant nephrotic syndrome (NS) due to focal segmental glomerulosclerosis (FSGS).1–3 Several studies have reported that LDL-A may be effective for non-FSGS or for diabetic nephropathy.4–6 Minimal change disease (MCD) often causes NS not only in children but also in adults.7–9 Renal insufficiency occurs in approximately 20–30% of patients with MCD.7 In MCD, it is often difficult to control effective circulating plasma volume and causes acute kidney injury (AKI), and when diuretics are not effective and the respiratory condition of patients worsens, patients require acute renal replacement therapy (ARRT) such as hemodialysis (HD) or the extracorporeal ultrafiltration method (ECUM). Furthermore, even if we treat patients with NS due to MCD with corticosteroids, which are the first-line therapy for MCD, some patients cannot discontinue ARRT because their NS and AKI are prolonged. The effectiveness of LDL-A is due to reducing serum low-density lipoprotein. However, LDL-A may also have various other benefits.1–6 We hypothesized that LDL-A could improve AKI caused by NS due to MCD and may help patients discontinue ARRT from these cases. We report two patients who had to undergo ARRT because their AKI was caused by MCD-induced NS, and who received LDL-A at Nippon Medical School Chiba Hokusoh Hospital.
Cases Presentation
We report two patients who were treated at Nippon Medical School Chiba Hokusoh Hospital between September 2017 and March 2019. We obtained written informed consent from the patients to publish this case series and accompanying images. A 49-year-old Japanese woman and a 71-year-old Japanese man with AKI caused by NS due to MCD underwent HD or ECUM to control their fluid volume and to treat their renal function failure. Tables 1 and 2 show the patient characteristics of a 49-year-old Japanese woman and a 71-year-old Japanese man, respectively, at the time of hospitalization. The female patient’s vital sing on the admission was Consciousness, Blood pressure 126/84 mmHg, Pulse 95/min, Respiratory rate of 17/min with an O2 saturation of 95%; room air, and the male patient’s vital sing on the admission was Consciousness, Blood pressure 142/82 mmHg, Pulse 76/min, Respiratory rate of 12/min with an O2 saturation of 95%; O2 6 L/min. The female patient’s post medical history was allergic dermatitis, and the male patient’s medical histories were hypertension, hepatitis B, vasospastic angina, atrial fibrillation, complete atrioventricular block, asbestosis, hypothyroidism and prostatic hypertrophy. The female was no medication, and the male patient’s medication were verapamil 240 mg/day, cilnidipine 10 mg/day, benidipine 8 mg/day, nicorandil 30 mg/day, trichlormethiazide 1mg/day, rosuvastatin 2.5 mg/day, lansoprazole 15 mg/day, furosemide 20mg/day, epinastine 20 mg/day and silodosin 8 mg/day. We performed renal biopsy on them for diagnosis the hospitalization day 12 and the hospitalization day 7, respectively. The results of the patients were minor glomerular abnormalities, MCD compatible; the number of glomeruli of their renal biopsy specimens were 12, and those included 2 and 3 global sclerosis, respectively, and those did not include segmental sclerosis.
Table 1.
Laboratory Findings at the Time of Admission: A 49-Year-Old Japanese Woman
| Urinalysis | Biochemical | Serology | |||
|---|---|---|---|---|---|
| Protein | (4+) | AST | 19 IU/L | CRP | 5.96 mg/dL |
| Glucose | (-) | ALT | 11 IU/L | IgG | 466 mg/dL |
| Occult blood | (2+) | T-Bil | 0.3 mg/dL | IgA | 256 mg/dL |
| LDH | 265 IU/L | IgM | 104 mg/dL | ||
| Sediment | CK | 63 IU/L | CH50 | 55 U/mL | |
| RBC | 10–19/HPF | TP | 3.8 g/dL | C3 | 178 mg/dL |
| WBC | 5–9/HPF | Alb | 1.5 g/dL | C4 | 52.1 mg/dL |
| Casts | 30–99/HPF | BUN | 32.2 mg/dL | Anti-nuclear Ab | <40 |
| Cre | 1.81 mg/dL | PR3-ANCA | (-) | ||
| Urinary protein excretion | 9.6 g/day | T-Cho | 550 mg/dL | MPO-ANCA | (-) |
| FENa | 3.36 | LDL-Cho | 423 mg/dL | Anti-GBM Ab | (-) |
| FEUN | 12.2% | TG | 315 mg/dL | HBs Ag | (-) |
| Na | 137 mEq/L | HCV Ab | (-) | ||
| Complete blood count | K | 4.7 mEq/L | TPHA | (-) | |
| WBC | 12,030/µL | Cl | 106 mEq/L | RPR | (-) |
| RBC | 469×10^4/µL | Glucose | 96 mg/dL | ||
| Hb | 11.0 g/dL | Selectivity Index | 0.14 | ||
| Hct | 34.20% | ||||
| Plt | 38.5×10^4/µL |
Abbreviations: RBC, red blood cell; WBC, white blood cell; FENa, fractional excretion of sodium; FEUN, fraction excretion of urea nitrogen; Hb, hemoglobin; Hct, hematocrit; Plt, platelet; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T-Bil, total- bilirubin; LDH, lactate dehydrogenase; CK, creatine kinase; TP, total protein; Alb, albumin; BUN, blood urea nitrogen; Cre, creatinine; T-Cho, total-cholesterol; LDL-Cho, low-density lipoprotein cholesterol; TG, triacylglycerol; CRP, C-reactive protein; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; Ab, antibodies; PR3-ANCA, proteinase-3-anti-neutrophil cytoplasmic antibodies; MPO-ANCA, myeloperoxidase-anti-neutrophil cytoplasmic antibodies; Anti-GBM, anti-glomerular basement membrane; HBs Ag, hepatitis B surface antigen; HCV, hepatitis C virus; TPHA, treponema pallidum hemagglutination test; RPR, rapid plasma reagin.
Table 2.
Laboratory Findings at the Time of Admission: A 71-Year-Old Japanese Man
| Urinalysis | Biochemical | Serology | |||
|---|---|---|---|---|---|
| Protein | (4+) | AST | 33 IU/L | CRP | 0.64 mg/dL |
| Glucose | (±) | ALT | 16 IU/L | IgG | 522 mg/dL |
| Occult blood | (2+) | T-Bil | 0.2 mg/dL | IgA | 125 mg/dL |
| LDH | 441 IU/L | IgM | 545 mg/dL | ||
| Sediment | CK | 421 IU/L | CH50 | 37 U/mL | |
| RBC | 5–9/HPF | TP | 4.5 g/dL | C3 | 114 mg/dL |
| WBC | 5–9/HPF | Alb | 1.9 g/dL | C4 | 35.5 mg/dL |
| Casts | 30–99/HPF | BUN | 43.6 mg/dL | Anti-nuclear Ab | <40 |
| Cre | 2.05 mg/dL | PR3-ANCA | (-) | ||
| Urinary protein excretion | 9.9 g/day | T-Cho | 304 mg/dL | MPO-ANCA | (-) |
| FENa | 6.18 | LDL-Cho | 191 mg/dL | Anti-GBM Ab | (-) |
| FEUN | 8.7% | TG | 162 mg/dL | HBs Ag | (-) |
| Na | 129 mEq/L | HCV Ab | (-) | ||
| Complete blood count | K | 4.7 mEq/L | TPHA | (-) | |
| WBC | 8200/µL | Cl | 99 mEq/L | RPR | (-) |
| RBC | 494×10^4/µL | Glucose | 114 mg/dL | ||
| Hb | 16.1 g/dL | Selectivity Index | 0.11 | ||
| Hct | 43.6% | ||||
| Plt | 25.1×10^4/µL |
Abbreviations: RBC, red blood cell; WBC, white blood cell; FENa, fractional excretion of sodium; FEUN, fraction excretion of urea nitrogen; Hb, hemoglobin; Hct, hematocrit; Plt, platelet; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T-Bil, total- bilirubin; LDH, lactate dehydrogenase; CK, creatine kinase; TP, total protein; Alb, albumin; BUN, blood urea nitrogen; Cre, creatinine; T-Cho, total-cholesterol; LDL-Cho, low-density lipoprotein cholesterol; TG, triacylglycerol; CRP, C-reactive protein; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; Ab, antibodies; PR3-ANCA, proteinase-3-anti-neutrophil cytoplasmic antibodies; MPO-ANCA, myeloperoxidase-anti-neutrophil cytoplasmic antibodies; Anti-GBM, anti-glomerular basement membrane; HBs Ag, hepatitis B surface antigen; HCV, hepatitis C virus; TPHA, treponema pallidum hemagglutination test; RPR, rapid plasma reagin.
The respiratory status of these patients worsened after admission to the hospital due to poor control of circulatory dynamics caused by AKI; their urinary volume (UV) also decreased, and their fluid volume and body weight increased. Therefore, patient one and patient two each underwent ARRT two days and one day after hospital admission, respectively. Corticosteroid therapy was also initiated to treat NS one and eight days after hospital admission. Although these patients received ARRT and corticosteroids to treat AKI and MCD diagnosed by renal biopsy, their AKI and MCD did not improve sufficiently; their UV also did not clearly increase, and hypoalbuminemia was prolonged. We decided to initiate LDL-A treatment for these patients as an additional treatment modality because their total serum cholesterol levels were high at the time of admission. The man already treated by rosuvastatin at the admission, and was not so high LDL levels but high total serum cholesterol levels, over 300 mg/dL. The female had not treated by cholesterol lowering medication at the admission, but she additionally treated by atorvastatin to hyper LDL levels. Both patients did not treat by human albumin supplement therapy through all courses. LDL-A treatment was initiated during corticosteroid treatment for 21 and 24 days for the female and male patient, respectively; the male patient received plasma exchange only once because he tested positive for serum cryoglobulin before we obtained the renal biopsy result. We then discontinued plasma exchange because we did not consider cryoglobulinemic vasculitis from the renal biopsy result.
LDL-A was performed using hollow polysulfone fibers (Sulflux FP-05®; Kaneka Co. Ltd., Osaka, Japan) as the plasma separator and a dextran sulfate cellulose column (Liposorber LA-15; Kaneka Co. Ltd., Osaka, Japan) as the LDL absorber.4 Approximately 3000–4000 mL of plasma was treated (nearly equal to body weight (kg)/13 × (1 - hematocrit)).
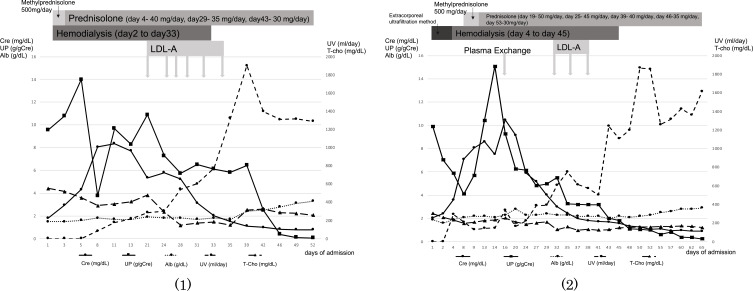
Despite therapy with corticosteroids for AKI and NS due to MCD, the fluid volume and renal function in these patients were not controlled before LDL-A treatment. After the additional LDL-A treatment, their fluid volume control, renal function, and urinary protein began to gradually improve. Figure 1 shows the clinical courses and changes in clinical status in (1) a 49-year-old Japanese woman and (2) a 71-year-old Japanese man. After the female patient had received LDL-A six times, UV exceeded 1300 mL/day and her serum albumin (Alb) was increased; thus, she was able to discontinue ARRT. After the male patient had received LDL-A three times, UV was improved and serum Alb was increased; thus, he was able to discontinue ARRT. Moreover, after LDL-A treatment, urinary protein was improved in both patients, and they achieved remission of MCD; therefore, they were discharged on the 54th and 67th day, respectively, after admission.
Figure 1.
Clinical course of patients: (1) a 49-year-old Japanese woman and (2) a 71-year-old Japanese man.
Abbreviations: LDL-A, low-density lipoprotein apheresis; Cre, serum creatinine; UP, urine protein; Alb, serum albumin; UV, urine volume; T-cho, serum total cholesterol.
Discussion
LDL-A was developed as a therapy for familial hypercholesterolemia, but it has been generally used for the treatment of drug-resistant NS that is specifically due to FSGS; LDL-A is used in this capacity in Japan as well.1–3 On the contrary, it has been reported that the effectiveness of LDL-A is not significantly different between FSGS and non-FSGS4 and that LDL-A effectively decreases proteinuria and podocyte excretion,5 which affects survival extension and renal function in diabetic nephropathy patients.6
The effectiveness of LDL-A lies in reducing serum low-density lipoprotein; LDL-A may also have various other benefits.1–6 Muso et al proposed that LDL-A improves hypercoagulability and renal hemodynamics and that LDL-A contributes to the therapeutic effect of antiproteinuric drugs such as corticosteroids.4 The urine volume in both patients was increased after additional LDL-A treatment because LDL-A might have improved their renal hemodynamics by reducing vasoconstrictive eicosanoids such as thromboxane A2 and by increasing prostaglandin I2.4 We also used corticosteroids as a first-line therapy for these patients with NS undergoing ARRT; we found that LDL-A might improve the bioavailability of drugs and treatment effects.4 Furthermore, Muso et al proposed that LDL-A initiated within eight weeks after the onset of NS may be more effective in the treatment and recovery of a patient’s nephrotic condition compared with when LDL-A is given more than eight weeks after NS onset.4 We initiated LDL-A treatment in both patients within eight weeks of NS onset. It is possible that both patients recovered effectively after they had received LDL-A because of this early intervention.
MCD often causes NS not only in children but also in adults, including those over the age of 60; MCD is seen in 10–15% of adult cases of primary NS.7–9 In MCD patients, renal function failure occurs in approximately 20–30% of cases because the filtration function of water and solutes is damaged due to foot process fusion and because AKI complicated by MCD is associated with significant morbidity and mortality.7 The total length of glomerular epithelial slit pores is reduced by the fusion of foot processes in MCD, which causes a reduction of glomerular capillary permeability to water and small solutes.10 The risk factors of acute renal failure in patients with MCD are high blood pressure, high serum cholesterol level, and severe hypoalbuminemia.11 Both patients in these cases had high serum cholesterol levels and severe hypoalbuminemia, which may have caused AKI.
Some studies have reported that endothelin 1 (ET-1) levels in renal tissue and in the urine of patients with MCD are elevated.12,13 ET-1 is known to be one of the most potent vasoconstrictors that affect renal vasculature. As a result, ET-1 decreases blood flow and GFR.11 Therefore, ET-1 may cause AKI in patients with NS due to MCD.11 Furthermore, one study reported that a single treatment with LDL-A decreased the plasma ET-1 level.14 LDL-A may improve AKI with NS due to MCD via a decrease in ET-1, although the pathophysiology of AKI in patients with MCD who require dialysis is still unclear.11
Steroid treatment is the first-line therapy for MCD.8 Other immunosuppressive agents such as cyclosporine, cyclophosphamide, mycophenolate mofetil, and tacrolimus are used as a second-line therapy for MCD.8 However, these drugs increase the risk for infection in these patients, and it has been reported that acute renal failure during the course of NS may increase the risk of infection.8 If patients require ARRT due to AKI caused by MCD, they also require vascular access, such as temporary catheter placement, through which they can receive ARRT without concomitant hemodialysis. Central venous catheter-related bloodstream infections are associated with compromised immune function;15 thus, it is possible that therapies such as corticosteroids increase the patients’ risk for catheter-related bloodstream infections. Although it is not completely understood how rituximab controls MCD,16 it has recently been used in some cases for the treatment of steroid-dependent MCD.9,16,17 In Japan, rituximab has been effectively used for childhood-onset, complicated, frequently relapsing NS, and steroid-dependent NS.18 Furthermore, even in patients with adult-onset NS, rituximab is as effective and safe as in childhood-onset NS.19,20 However, a concern of long-term rituximab therapy is the development of low immunoglobulin levels.21 LDL-A therapy may thus be advantageous in patients undergoing ARRT for AKI caused by NS due to MCD because they do not require new vascular access for only LDL-A and because LDL-A itself is not known to increase the risk of infection. One report states that LDL-A is effective for patients with rituximab-resistant MCD.22
These cases suggest that even if patients requiring ARRT caused by MCD do not improve sufficiently with corticosteroids as a first-line therapy, the early additive indication of LDL-A in these patients is effective and allows discontinuance of ARRT and remission of NS due to MCD because of the effect of LDL-A such as improving hypercoagulability and renal hemodynamics and contributing to the therapeutic effect of corticosteroids. However, it is not sure that their improvements in these cases were actually caused by the additive indication of LDL-A, because it is possible that they improved due to only corticosteroids therapy without the additive indication LDL-A. To understand the true effects of LDL-A for AKI due to MCD, we should certainly conduct an additional research such as comparing with control.
This case series had certain limitations to prove our hypothesis that LDL-A could improve AKI caused by NS due to MCD and may help patients discontinue ARRT. Firstly, it was experienced at a single center, and therefore the number of participants was limited to only two patients. Secondly, we could not compare with control patients who did not be added LDL-A therapy to corticosteroids therapy. Thus, we need additional researches to prove the hypothesis.
Conclusion
We achieved remission of NS due to MCD after LDL-A treatment, as seen in other reports. Furthermore, it is possible that early additional LDL-A is effective for patients with AKI and drug-resistant NS due to MCD who are undergoing ARRT, and may help patients discontinue ARRT and not increase the risk of infection. We suggest early additional LDL-A for AKI caused by NS due to MCD as an alternative therapy.
Acknowledgments
The authors thank all the participants and the staffs of Nippon Medical School Chiba Hokusoh hospital.
Funding Statement
None.
Ethics Approval and Consent to Participate
Not required because this is a case series.
Consent for Publication
We obtained written informed consent from the patients to publish this case report and accompanying images. A copy of the written consent is available for review by the editor of this journal. However, our institution does not require ethics committee permission for case reporting.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Kobayashi S. Applications of LDL-apheresis in nephrology. Clin Exp Nephrol. 2008;12(1):9–15. doi: 10.1007/s10157-007-0003-8 [DOI] [PubMed] [Google Scholar]
- 2.Raina R, Krishnappa V. An update on LDL apheresis for nephrotic syndrome. Pediatr Nephrol. 2019;34(10):1655–1669. doi: 10.1007/s00467-018-4061-9 [DOI] [PubMed] [Google Scholar]
- 3.Makino H, Tamanaha T, Harada-Shiba M. LDL apheresis in Japan. Transfus Apher Sci. 2017;56(5):677–681. doi: 10.1016/j.transci.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 4.Muso E, Mune M, Hirano T, et al. Immediate therapeutic efficacy of low-density lipoprotein apheresis for drug-resistant nephrotic syndrome: evidence from the short-term results from the POLARIS study. Clin Exp Nephrol. 2015;19(3):379–386. doi: 10.1007/s10157-014-0996-8 [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Kawagoe Y, Ogawa H, et al. Effect of low-density lipoprotein apheresis on urinary protein and podocyte excretion in patients with nephrotic syndrome due to diabetic nephropathy. Am J Kidney Dis. 2005;45(1):48–53. doi: 10.1053/j.ajkd.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 6.Sato E, Amaha M, Nomura M, Matsumura D, Ueda Y, Nakamura T. LDL-apheresis contributes to survival extension and renal function maintenance of severe diabetic nephropathy patients: a retrospective analysis. Diabetes Res Clin Pract. 2014;106(2):241–246. doi: 10.1016/j.diabres.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 7.Meyrier A, Niaudet P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018;94(5):861–869. doi: 10.1016/j.kint.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 8.Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2(3):445–453. doi: 10.2215/CJN.03531006 [DOI] [PubMed] [Google Scholar]
- 9.Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332–345. doi: 10.2215/CJN.05000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohman SO, Jaremko G, Bohlin AB, Berg U. Foot process fusion and glomerular filtration rate in minimal change nephrotic syndrome. Kidney Int. 1984;25(4):696–700. doi: 10.1038/ki.1984.76 [DOI] [PubMed] [Google Scholar]
- 11.Chen CL, Fang HC, Chou KJ, et al. Increased endothelin 1 expression in adult-onset minimal change nephropathy with acute renal failure. Am J Kidney Dis. 2005;45(5):818–825. doi: 10.1053/j.ajkd.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 12.Wolf SC, Smoltczyk H, Brehm BR, Erley CM, Risler T. Endothelin-1 and endothelin-3 levels in different types of glomerulonephritis. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S482–5. doi: 10.1097/00005344-199800001-00138 [DOI] [PubMed] [Google Scholar]
- 13.Murer L, Zacchello G, Basso G, et al. Immunohistochemical distribution of endothelin in biopsies of pediatric nephrotic syndrome. Am J Nephrol. 1994;14(3):157–161. doi: 10.1159/000168707 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Ushiyama C, Osada S, Inoue T, Shimada N, Koide H. Effect of low-density lipoprotein apheresis on plasma endothelin-1 levels in diabetic hemodialysis patients with arteriosclerosis obliterans. J Diabetes Complications. 2003;17(6):349–354. doi: 10.1016/S1056-8727(02)00171-X [DOI] [PubMed] [Google Scholar]
- 15.Cheng S, Xu S, Guo J, et al. Risk factors of central venous catheter-related bloodstream infection for continuous renal replacement therapy in kidney intensive care unit patients. Blood Purif. 2019;48(2):175–182. doi: 10.1159/000495024 [DOI] [PubMed] [Google Scholar]
- 16.Madanchi N, Bitzan M, Takano T. Rituximab in minimal change disease: mechanisms of action and hypotheses for future studies. Can J Kidney Health Dis. 2017;13(4):2054358117698667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munyentwali H, Bouachi K, Audard V, et al. Rituximab is an efficient and safe treatment in adults with steroid-dependent minimal change disease. Kidney Int. 2013;83(3):511–516. doi: 10.1038/ki.2012.444 [DOI] [PubMed] [Google Scholar]
- 18.Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset refractory nephrotic syndrome (RCRNS) study group. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384(9950):1273–1281. doi: 10.1016/S0140-6736(14)60541-9 [DOI] [PubMed] [Google Scholar]
- 19.Iwabuchi Y, Miyabe Y, Makabe S, et al. Comparison of the response of frequently relapsing steroid-dependent minimal change nephrotic syndrome to rituximab therapy between childhood-onset and adult-onset disease. Medicine (Baltimore). 2018;97(42):e12704. doi: 10.1097/MD.0000000000012704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsuno T, Masuda T, Saito S, et al. Therapeutic efficacy of rituximab for the management of adult-onset steroid-dependent nephrotic syndrome: a retrospective study. Clin Exp Nephrol. 2019;23(2):207–214. doi: 10.1007/s10157-018-1630-y [DOI] [PubMed] [Google Scholar]
- 21.Bruchfeld A, Benedek S, Hilderman M, Medin C, Snaedal-Jonsdottir S, Korkeila M. Rituximab for minimal change disease in adults: long-term follow-up. Nephrol Dial Transplant. 2014;29(4):851–856. doi: 10.1093/ndt/gft312 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Tsukamoto T, Muso E. Rituximab-resistant nephrotic syndrome with successful induction of remission by low-density lipoprotein apheresis. Ther Apher Dial. 2017;21(3):295–296. doi: 10.1111/1744-9987.12561 [DOI] [PubMed] [Google Scholar]