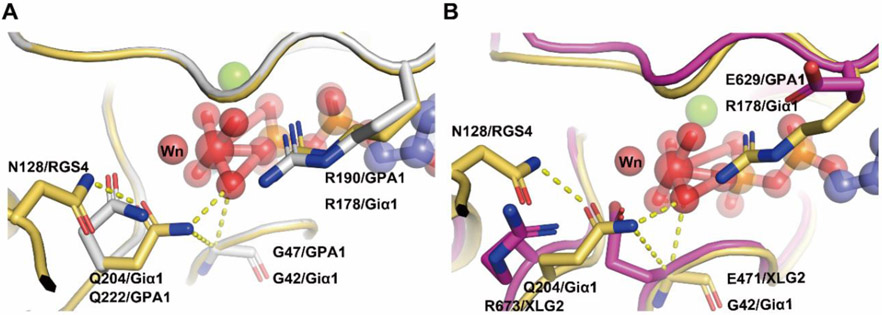
Figure 3. Interactions between the catalysis center of the Ras domain and critical residues of RGS proteins.
(A) The critical contact residues between Gαi1 and RGS4 (PDB 1AGR) are shown in light orange. Arg 178 (Rcat) is within hydrogen bonding distance of the leaving group β-γ bridge oxygen and Q204 (Qcat) is a hydrogen bond donor to a fluorine (or O–) Al substituent and accepts a hydrogen bond from the presumptive water nucleophile (Wn). The hydrogen bond network (yellow dashed lines) involving N128 (Asn thumb of RGS4), Qcat, G42 and the the γ phosphate (modeled by AlF4) orient Wn for nucleophilic attack and stabilize developing charge at the β-γ bridge leaving group oxygen. RGS4 residues Asn 128 constrain the conformation of Gαi1 Q204 (Qcat) to the pre-transition state conformation. AtGPA1 contains the same catalysis network (A) however the catalysis network was disrupted in XLG2 with the loss of the Glncat and Arg finger and replaced by R673 and E629 respectively (B). Grey: AtGPA1, Magenta: XLG2, Light orange: Giα1. The substrate GDP and AlF4 are shown as sticks and spheres. Main catalysis residues between Giα1, AtGPA1, XLG2 and RGS4 are highlighted as sticks. Wn: nucleophilic water.