Abstract
Purpose
To examine the impact of pre-diagnosis depressive symptoms and mental health-related quality of life (HRQOL) on survival among older patients with multiple myeloma (MM).
Methods
We performed a retrospective cohort study using the Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey data resource. Patients aged 65 years and older diagnosed with first primary MM between 1998 and 2014 were identified and presence of depressive symptoms was determined based on responses to 3 depression screening questions prior to MM diagnosis. Veterans-RAND-12 mental component scores (MCS) were analyzed to evaluate mental HRQOL. We used multivariable Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for risks of all-cause and cancer-specific mortality.
Results
Of 522 patients, mean (SD) age at diagnosis was 76.9 (6.1) years and 158 (30%) reported depressive symptoms. Patients with depressive symptoms had a higher number of comorbid conditions and nearly all (84%) scored below the median MCS. Pre-diagnosis depressive symptoms were not associated with all-cause (HR=1.01, 95% CI: 0.79–1.29) or cancer-specific mortality (HR=0.94, 95% CI: 0.69–1.28). MM patients scoring in the second MCS tertile (versus the highest tertile) had a modestly increased risk of all-cause (HR=1.19, 95% CI 0.91–1.55) and cancer-specific mortality (HR=1.17, 95% CI 0.86–1.60), but these estimates were not statistically significant.
Conclusion
Pre-diagnosis depressive symptoms and lower mental HRQoL did not impact survival among older MM patients. Highly prevalent depressive symptoms among older MM patients deserves clinical attention. Such efforts can inform clinicians in tailoring care for this vulnerable population.
Keywords: Multiple myeloma, cancer survival, depression, mental health, elderly
Introduction
Multiple myeloma (MM) is a plasma-cell malignancy characterized with proliferation of plasma cells in the bone marrow, monoclonal protein in the blood and urine, and end-organ dysfunction leading to significant morbidity and mortality [1, 2]. Although a rare disease, MM accounts for 17% of blood cancers making it the second most common hematological malignancy in the United States (US) [3]. MM is a cancer of older adults with median age at diagnosis of 70 years [1]. Survival of MM patients has improved in recent years due to treatment advancements including autologous stem cell transplantation and novel agents such as thalidomide, lenalidomide, and bortezomib [1], and 5-year survival rates for MM are slightly over 50% [3].
Depression among older adults is a major public health problem that is often underreported and underdiagnosed [4, 5]. Prevalence of depression is reported to be between 1% and 16% among patients 65 years and older [6]. High prevalence of depression in older patients often presents with several comorbidities, which together can worsen quality of life, increase healthcare utilization, and lead to higher risk for suicide [5]. Depression is also more common among cancer patients, approximately four-times higher than average depression rates in the general population [7].
Depression and poor mental health-related quality of life (HRQOL) can detrimentally impact cancer outcomes through the biobehavioral effects of psychological stressors [8]. Depression can potentially worsen cancer progression through compromised cancer defense mechanisms and dysregulated oxidative stress [8]. Depression and poor mental health are also associated with poor medication adherence, worse quality of life, longer hospitalizations, and suboptimal treatment outcomes among cancer patients [8–12].
Previous studies relating depression and poor mental HRQOL with cancer outcomes report inconsistent findings with a paucity of evidence among MM patients. Among studies evaluating depression and depressive symptoms in cancer patients, some indicated up to a two-fold greater risk of mortality [13–16], whereas others found no association [17]. Conflicting trends were also observed in studies relating poor mental HRQOL and survival among cancer patients [18–20]. Furthermore, previous studies were heterogeneous in terms of patient populations, cancer types, and depression definition, which can limit generalizability to MM patients. Lastly, the majority of studies assessed depression and mental HRQOL after cancer diagnosis and sometimes after treatment initiation [19]. These assessments can be influenced by subsequent cancer or treatment-related factors, which have been shown to impact depression and HRQOL [11, 21]. To our knowledge, no previous study evaluated the association between pre-diagnosis depressive symptoms or poor mental HRQOL and survival among patients with MM.
To fill this knowledge gap, we analyzed data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare Health Outcomes Survey (MHOS). The SEER-MHOS data resource provides detailed clinical and HRQOL data for older Medicare beneficiaries in the US residing in regions covered by the SEER population-based cancer registries [22]. In addition, the SEER-MHOS data include specific questions regarding depression and mental HRQOL along with robust survival information of MM patients [22]. Our objective was to examine the impact of pre-diagnosis depressive symptoms and poor mental HRQOL on the survival of elderly MM patients.
Methods
Study design and population
We conducted a prospective cohort study utilizing the SEER-MHOS 1998 to 2014 data with information on vital status and cause of death through November 30th, 2016. SEER-MHOS is a robust data resource that links information from two datasets (SEER and the MHOS surveys) to provide insights about older cancer patients in the US [22]. The SEER program of the National Cancer Institute collects information on cancer incidence and survival covering 99% of 18 population-based cancer registries that represent about 35% of the US population [23]. The SEER program has information on demographics, clinical characteristics (cancer diagnoses, extent of disease, tumor markers, surgery and radiation) and survival [24]. MHOS is a survey of patient reported outcomes from a randomly selected sample of Medicare Advantage plan beneficiaries [22]. MHOS baseline surveys are distributed yearly to over 140,000 Medicare Advantage enrollees with a follow-up survey collected every 2 years for patients still enrolled in the same plan [25]. Response rates for first and follow-up MHOS surveys are approximately 60% [25] and can be completed by the patient or by a proxy [22]. Information in MHOS includes self-reported demographics, socioeconomic status, comorbidities, functional status, and measures of HRQOL [22].
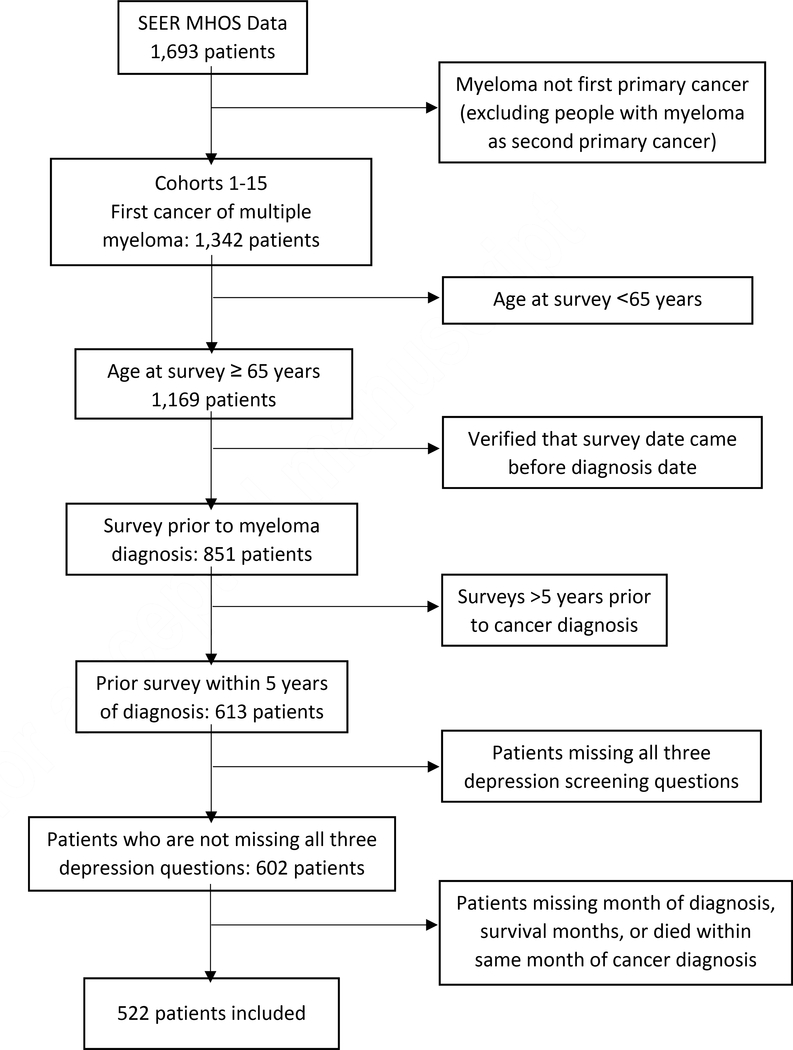
This study utilized data from SEER-MHOS cohorts between 1998 and 2014, composed of more than 140,000 cancer survivors [25]. Study population and inclusion/exclusion criteria are outlined in Figure 1. We included patients that were: (i) 65 years and older at time of SEER-MHOS survey; (ii) diagnosed with first primary MM between 1998 and 2014; and (iii) provided a minimum of one SEER-MHOS survey within 5 years prior to MM diagnosis. Among patients who completed multiple pre-diagnosis surveys, we examined the most recent survey prior to MM diagnosis. Patients were excluded if they were missing: all 3 depression screening questions, valid MM diagnosis date, and/or information on post-diagnosis survival months. Patients were also excluded if they took the survey or died in the same month of MM diagnosis. This study was determined to be exempt by the institutional review board of the University of Illinois at Chicago.
Figure 1:
Consort Diagram
Exposure Measures
Depressive symptoms and mental HRQOL were collected from SEER-MHOS surveys prior to MM diagnosis. Presence of pre-diagnosis depressive symptoms was based on results from Diagnostic Interview Schedule (DIS) 3-item questionnaire, administered at least 1 month prior to MM diagnosis. Presence of depressive symptoms was defined as affirmative response to one of the 3 following questions: (1) “In the past year, have you had 2 weeks of depression? (Yes/No);” (2) “In the past year, have you had depression much of the time? (Yes/No);” (3) “In the past 2 years, have you had depression most of the time? (Yes/No).” Previous studies defining depression in SEER-MHOS data used similar DIS criteria with one study including a mental component summary (MCS) threshold of <42 for characterizing depressive symptoms [26, 27]. While no information is available on the predictive performance of DIS in Medicare patients, a slightly different version of DIS demonstrated 83% to 94% sensitivity and above 90% specificity; [28] and a 2-item version of DIS studied in elderly patients with mean age of 53 years showed 96% sensitivity, 57% specificity, and area under the Receiver Operating Characteristic (ROC) curve of 0.82 [29]. Since there is no uniform definition for depressive symptoms in Medicare patients, sensitivity analyses were conducted to vary exposure over a plausible range and assess robustness of our findings. In one analysis, depressive symptoms definition was restricted to affirmative response to items 1 and 2 of DIS to improve recency of the exposure. Another sensitivity analysis was carried out by excluding proxy surveys.
Mental HRQOL was determined using the Mental Component Summary (MCS) score based on the Short Form Health Survey (SF-36) and Veterans Rand 12 Health Survey (VR-12). VR-12 is a patient-reported general health measure used to assess a patient’s overall perspective of their health [30]. VR-12 replaced SF-36 starting 2006 in SEER-MHOS data. Results from SF-36 and VR-12 were bridged using a published algorithm to account for missing data and make scores comparable across all SEER-MHOS cohorts [31]. Subscale scores for 8 physical and mental HRQOL domains were collected including physical functioning (PF), role limitation due to physical health problems (RP), bodily pain (BP), general health perception (GH), mental health (MH), role limitation due to personal or emotional problems (RE), social functioning (SF), and vitality or energy (VT). Group averages for HRQOL domains lie in 0 to 100 range [30]. Mental and physical component summary scores (MCS-12 and PCS-12) were computed based on subscale scores from mental and physical HRQOL domains. Higher MCS, PCS, and subscale scores signify better HRQOL [30].
Outcome Measures
The primary outcomes were cancer-specific and all-cause mortality using survival information documented by the SEER registries. Survival was defined as the proportion of MM patients alive subsequent to MM diagnosis as of November 30th, 2016.
Statistical Analysis
Patient demographics, socioeconomic status, self-reported comorbidities, MM clinical characteristics, and survey characteristics were collected from SEER-MHOS survey prior to MM diagnosis. Demographic and socioeconomic data included age, race, sex, education, and marital status. Number of comorbidities was obtained by totaling responses to 15 self-reported comorbidity questions. Possible comorbidities included the following: heart conditions (hypertension, angina or coronary artery disease, congestive heart failure, acute myocardial infarction, other heart conditions), stroke, pulmonary conditions (emphysema, asthma, COPD), Crohn’s Disease, Ulcerative Colitis, Inflammatory Bowel Disease, diabetes, arthritis, and sciatica. If a patient was missing data on any comorbidity, then number of comorbid conditions was considered missing. Number of heart, pulmonary and cardiovascular conditions were computed as appropriate with aggregates deemed missing if responses to any of the individual comorbid conditions were missing. Survey characteristics encompassed number of months from MHOS survey to MM diagnosis, survey disposition (mail/telephone), and survey respondent (patient/proxy).
Self-reported general health, MCS-12, PCS-12, and HRQOL domains were also described. Bivariate analyses were conducted to compare demographics, clinical characteristics, and HRQOL measures for patients with and without pre-diagnosis depressive symptoms. Chi square and Fisher tests were used for categorical data and Wilcoxon rank sum test for continuous data with a 0.05 significance level.
Cox proportional hazards models were used to estimate crude and adjusted HR and 95% CI for the association between each of pre-diagnosis depressive symptoms and mental HRQOL with risks of all-cause and cancer-specific mortality. Estimates from Cox models were adjusted for sex, race, age, education, categories of number of comorbidities, marital status, PCS score (tertiles), year of diagnosis, and smoking status. Competing risks were accounted for in models for cancer-specific mortality by estimating cause-specific hazards and censoring at the time of other-cause death [32]. All-cause mortality estimates were calculated in the absence of competing risks. Possible interaction between depressive symptoms and mental component scores were evaluated in sensitivity analyses including interaction terms and stratification. The proportional hazards assumption was evaluated using interaction of log-follow up time and graphical assessments based on scaled Schoenfeld residuals. No evidence was observed to suggest violation of the proportionality assumption. All analysis was performed in SAS version 9.4 (Cary, NC).
Results
Demographics and clinical characteristics are reported in Table 1. Of the 522 patients diagnosed with first primary MM between 1998 and 2014 and included in the final analytic sample, 158 (30%) reported depressive symptoms prior to MM diagnosis. Patients had a mean (SD) age of 77 (6) years, with most patients (59%) at age 75 years or older. Sixty percent of patients identified as white and 18% were black. Overall, patients reported an average of 2.7 comorbid conditions with 74% reporting at least 1 heart condition.
Table 1:
Demographics and clinical characteristics
| Characteristics | Total (n=522) | Depressive Symptoms (n=158) | No Depressive Symptoms (n=364) | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age at diagnosis Mean, (SD) | 76.9 | 6.1 | 76.0 | 6.6 | 75.1 | 6.0 | 0.13 |
| 65 – 74 | 215 | 41.2% | 61 | 38.6% | 154 | 42.3% | |
| 75 – 84 | 244 | 46.7% | 71 | 44.9% | 173 | 47.5% | |
| 85+ | 63 | 12.1% | 26 | 16.5% | 37 | 10.2% | |
| Race/ Ethnicity | 0.35 | ||||||
| White | 312 | 59.8% | 87 | 55.1% | 225 | 61.8% | |
| Black or African American | 95 | 18.2% | 32 | 20.3% | 63 | 17.3% | |
| Other | 115 | 22.0% | 39 | 24.7% | 76 | 20.9% | |
| Sex | 0.02 | ||||||
| Male | 259 | 49.6% | 66 | 41.8% | 193 | 53.0% | |
| Female | 263 | 50.4% | 92 | 58.2% | 171 | 47.0% | |
| Marital status | <0.01 | ||||||
| Married | 287 | 55.0% | 68 | 43.0% | 219 | 60.2% | |
| Not married | 225 | 43.1% | 86 | 54.4% | 139 | 38.2% | |
| Education | <0.01 | ||||||
| Less than high school | 161 | 30.8% | 70 | 44.3% | 91 | 25.0% | |
| High school graduate or GED | 157 | 30.1% | 48 | 30.4% | 109 | 29.9% | |
| College or above | 190 | 36.4% | 34 | 21.5% | 156 | 42.9% | |
| Smoking status | <0.01 | ||||||
| Yes (every day or some days) | 49 | 9.4% | 27 | 17.1% | 22 | 6.0% | |
| Not at all | 399 | 76.4% | 111 | 70.3% | 288 | 79.1% | |
| Unknown | 74 | 14.2% | 20 | 12.7% | 54 | 14.8% | |
| Number of comorbid conditionsa Mean, (SD) | 2.7 | 2.0 | 3.5 | 2.1 | 2.4 | 1.8 | <0.01 |
| 0–2 | 248 | 47.5% | 56 | 35.4% | 192 | 52.7% | |
| 3 or more | 214 | 41.0% | 80 | 50.6% | 134 | 36.8% | |
| Heart conditionsb | 0.03 | ||||||
| At least 1 heart condition | 384 | 73.6% | 126 | 79.7% | 258 | 70.9% | |
| No heart conditions | 137 | 26.2% | 31 | 19.6% | 106 | 29.1% | |
| Stroke | |||||||
| Yes | 48 | 9.2% | 19 | 12.0% | 29 | 8.0% | 0.13 |
| No | 464 | 88.9% | 135 | 85.4% | 329 | 90.4% | |
| Cardiovascular conditionsc | 0.01 | ||||||
| At least 1 cardiovascular condition | 387 | 74.1% | 128 | 81.0% | 259 | 71.2% | |
| No cardiovascular conditions | 133 | 25.5% | 29 | 18.4% | 104 | 28.6% | |
| Diabetes | 0.03 | ||||||
| Yes | 123 | 23.6% | 47 | 29.7% | 76 | 20.9% | |
| No | 392 | 75.1% | 109 | 69.0% | 283 | 77.7% | |
| Arthritis | <0.01 | ||||||
| Arthritis | 290 | 55.6% | 107 | 67.7% | 183 | 50.3% | |
| No arthritis | 229 | 43.9% | 49 | 31.0% | 180 | 49.5% | |
| Asthma/COPD/Emphysema | <0.01 | ||||||
| Yes | 73 | 14.0% | 34 | 21.5% | 39 | 10.7% | |
| No | 441 | 84.5% | 121 | 76.6% | 320 | 87.9% | |
| Sciatica | <0.01 | ||||||
| Yes | 116 | 22.2% | 51 | 32.3% | 65 | 17.9% | |
| No | 397 | 76.1% | 104 | 65.8% | 293 | 80.5% | |
| Year of Multiple Myeloma Diagnosis | 0.02 | ||||||
| 1998–2005 | 207 | 39.7% | 66 | 41.8% | 141 | 38.7% | |
| 2006–2010 | 155 | 29.7% | 34 | 21.5% | 121 | 33.2% | |
| 2011–2013 | 160 | 30.7% | 58 | 36.7% | 102 | 28.0% | |
| Number of months from survey to diagnosis Mean, (SD) | 23.1 | 18.1 | 22.2 | 16.8 | 23.6 | 18.7 | 0.42 |
| MHOS survey disposition | 0.54 | ||||||
| 450 | 86.2% | 134 | 84.8% | 316 | 86.8% | ||
| Telephone | 72 | 13.8% | 24 | 15.2% | 48 | 13.2% | |
| Who completed survey | 0.01 | ||||||
| Patient | 416 | 79.7% | 112 | 70.9% | 304 | 83.5% | |
| Proxy | 70 | 13.4% | 30 | 19.0% | 40 | 11.0% | |
Possible patient reported comorbidities include hypertension, angina or coronary artery disease, congestive heart failure, acute myocardial infarction, other heart conditions, stroke, pulmonary conditions (emphysema, asthma, COPD), Crohn’s Disease, Ulcerative Colitis, or Inflammatory Bowel Disease, diabetes, arthritis, sciatica. If patient is missing data on any comorbidity, then number of comorbid conditions is considered missing
Possible patient reported heart conditions include hypertension, angina or coronary artery disease, congestive heart failure, acute myocardial infarction, other heart conditions
Include heart conditions and stroke
Note: Column percentages do not sum to 100% for some variables due to missing data
Fewer patients with pre-diagnosis depressive symptoms were married (43% vs 60%, P<0.01) and reported lower rates of college level education or above (22% vs 43%, P<0.01) compared to patients without depressive symptoms. Mean (SD) number of self-reported comorbidities was 3.5 (2.1) in patients with depressive symptoms compared to 2.4 (1.8) comorbidities in the comparator group (P<0.01). Surveys were mostly completed by patients (80%) and administered at average of 23 months prior to MM diagnosis; and survey responses were completed by proxy at higher rates among patients with depressive symptoms compared to those without depressive symptoms (P<0.01).
Results from general health, mental and physical HRQOL measures are described in Table 2. For HRQOL measures, the proportion of patients with depressive symptoms reporting poor or fair general health was more than double (52% vs 23%, P<0.01). Nearly all (84%) of patients with depressive symptoms scored below the median MCS-12 score, compared to 35% of the comparator group (P<0.01). Similar trends were observed in PCS-12 and HRQOL domain scores with significantly lower scores among patients with depressive symptoms. HRQOL domains differed between groups with and without depressive symptoms by 20 points or higher on average for PF, RP, MH, and RE (P<0.01).
Table 2:
General, mental and physical health-related quality of life
| Characteristics | Total (n=522) | Depressive Symptoms (n=158) | No Depressive Symptoms (n=364) | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| General Health | |||||||
| Poor or Fair | 164 | 31.4% | 82 | 51.9% | 82 | 22.5% | <0.01 |
| Good or above | 349 | 66.9% | 69 | 43.7% | 280 | 76.9% | |
| MCS-12 Median, (IQR) | 53.8 | 43.3–59.3 | 39.7 | 33.7–48.6 | 56.9 | 51.2–60.7 | <0.01 |
| MCS-12 Categories | |||||||
| Below or equal to median | 260 | 50.1% | 133 | 84.7% | 127 | 35.1% | <0.01 |
| Above median | 259 | 49.9% | 24 | 15.3% | 235 | 64.9% | |
| PCS-12 Median, (IQR) | 38.8 | 29.5–48.8 | 32.7 | 24.3–41.7 | 41.4 | 31.8–50.9 | <0.01 |
| PCS-12 Categories | |||||||
| Below or equal to median | 261 | 50.0% | 106 | 67.1% | 155 | 42.6% | <0.01 |
| Above median | 261 | 50.0% | 52 | 32.9% | 209 | 57.4% | |
| HRQL Domains | |||||||
| PF Median, (IQR) | 58.3 | 36.6–90.0 | 49.5 | 17.5–70.0 | 71.4 | 45.0–93.5 | <0.01 |
| RP Median, (IQR) | 50.0 | 0.5–100.0 | 9.8 | 0.0–53.0 | 75.0 | 9.8–100.0 | <0.01 |
| BP Median, (IQR) | 56.3 | 39.0–84.0 | 41.0 | 31.3–54.1 | 56.3 | 44.0–95.2 | <0.01 |
| GH Median, (IQR) | 61.5 | 40.0–77.0 | 46.0 | 37.5–61.5 | 61.5 | 52.0–82.0 | <0.01 |
| MH Median, (IQR) | 83.7 | 60.7–92.0 | 56.0 | 44.0–69.5 | 84.0 | 76.0–92.7 | <0.01 |
| RE Median, (IQR) | 100.0 | 33.3–104.1 | 33.3 | 0.0–75.1 | 100.0 | 66.7–104.1 | <0.01 |
| SF Median, (IQR) | 87.5 | 50.0–99.3 | 50.0 | 37.5–75.0 | 99.3 | 66.9–100.0 | <0.01 |
| VT Median, (IQR) | 54.6 | 40.0–79.3 | 40.9 | 30.0–50.0 | 59.7 | 45.9–79.3 | <0.01 |
IQR: Interquartile Range; PCS-12: Physical Component Summary score; MCS-12: Mental Component Summary score; VR-12: Veteran Rand 12-item questionnaire; HRQoL: Health-related Quality of Life; PF: Physical Functioning, RP: Role limitation due to physical health problems, BP: Bodily pain, GH: General health perception, MH: Mental health, RE: Role limitation due to personal or emotional problems, SF: Social functioning, VT: Vitality or energy
Results from Cox proportional hazards models for associations of pre-diagnosis depressive symptoms and MCS-12 with all-cause and cancer-specific mortality risks are reported in Table 3 and Table 4, respectively. Pre-diagnosis depressive symptoms were not associated with all-cause (HR 1.01, 95% CI: 0.79–1.29) or cancer-specific mortality (HR 0.94, 95% CI: 0.69–1.28). Myeloma patients scoring in the second MCS tertile (versus the highest tertile) showed a trend toward higher risk of all-cause (HR 1.19, 95% CI 0.91–1.55) and cancer-specific mortality (HR 1.17, 95% CI 0.86–1.60). However, these estimates were not statistically significant. No significant associations were observed when comparing the first MCS tertile to the third tertile in terms of all-cause (HR 1.07, 95% CI 0.80–1.43) and cancer-specific mortality (HR 0.98, 95% CI 0.68–1.40). Sensitivity analyses of 2-item DIS and proxy survey exclusion did not show substantively different results with respect to direction or statistical significance (Table S2). In sensitivity analyses stratifying the effects depressive symptoms and MCS tertiles on all-cause and cancer-specific mortality, there was no evidence suggesting significant interaction between pre-diagnosis depressive symptoms and MCS-12 (Tables S3 and S4).
Table 3:
All-cause and cancer-specific mortality for patients with depressive symptoms prior to multiple myeloma diagnosis
| All-Cause Mortality (total survival time=15,279 months, Events=363) | |||||||||||
| Covariate | Person Years | Events | Crude Model (n=522) | Minimally Adjusteda (n=522) | Fully Adjustedb (n= 506, Events=354c) | ||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| No depressive symptoms (reference) | 903.33 | 254 | 1 | - | - | 1 | - | - | 1 | - | - |
| Depressive symptoms | 369.92 | 109 | 1.04 | 0.83–1.30 | 0.74 | 1.04 | 0.82–1.30 | 0.76 | 1.01 | 0.79–1.29 | 0.94 |
| Cancer Specific Mortality (total survival time=15,279 months, Events=247) | |||||||||||
| Covariate | Person Years | Events | Crude Model (n=522) | Minimally Adjusteda (n=522) | Fully Adjustedb (n=506, Events=241c) | ||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| No depressive symptoms (reference) | 903.33 | 180 | 1 | - | - | 1 | - | - | 1 | - | - |
| Depressive symptoms | 369.92 | 67 | 0.90 | 0.68–1.20 | 0.48 | 0.90 | 0.67–1.19 | 0.45 | 0.94 | 0.69–1.28 | 0.70 |
adjusted for age, sex, race
adjusted for sex, race, age, education, categories of number of comorbidities, marital status, PCS score (tertiles), year of diagnosis, smoking status
Number of events for fully adjusted model was lower than crude and minimally adjusted models due to missing values in covariates included for adjustment.
Table 4:
All-cause and cancer-specific mortality for patients with poor mental health prior to multiple myeloma diagnosis
| All-Cause Mortality (total survival time=15,244 months, Events=360) | |||||||||||
| Covariate | Person years | Events | Crude Model (n=519) | Minimally Adjusteda (n=519) | Fully Adjustedb (n=503, Events=351c) | ||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| MCS-12 | |||||||||||
| Third tertile (reference) | 437.92 | 117 | 1 | - | - | 1 | - | - | 1 | - | - |
| Second tertile | 423.58 | 122 | 1.08 | 0.84–1.39 | 0.56 | 1.12 | 0.87–1.45 | 0.39 | 1.19 | 0.91–1.55 | 0.20 |
| First tertile | 408.83 | 121 | 1.10 | 0.85–1.42 | 0.47 | 1.12 | 0.87–1.45 | 0.38 | 1.07 | 0.80–1.43 | 0.65 |
| Cancer Specific Mortality (total survival time =15,244 months, events = 244) | |||||||||||
| Covariate | Person years | Events | Crude Model (n=519) | Minimally Adjusteda (n=519) | Fully Adjustedb (n=503, Events=238c) | ||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| MCS-12 | |||||||||||
| Third tertile (reference) | 437.92 | 84 | 1 | - | - | 1 | - | - | 1 | - | - |
| Second tertile | 423.58 | 87 | 1.07 | 0.79–1.45 | 0.66 | 1.10 | 0.81–1.48 | 0.55 | 1.17 | 0.86–1.60 | 0.32 |
| First tertile | 408.83 | 73 | 0.92 | 0.67–1.26 | 0.67 | 0.93 | 0.68–1.28 | 0.65 | 0.98 | 0.68–1.40 | 0.89 |
adjusted for age, sex, race
adjusted for sex, race, age, education, categories of number of comorbidities, marital status, PCS score (tertiles), year of diagnosis, smoking status
Number of events for fully adjusted model was lower than crude and minimally adjusted models due to missing values in covariates included for adjustment
MCS-12: Mental Component Summary score
Discussion
To our knowledge, this is the first study assessing the impact of pre-existing depressive symptoms and poor mental HRQOL on the survival of MM patients. We found a high prevalence of pre-diagnosis depressive symptoms in older patients prior to diagnosis with first primary MM. Patients with depressive symptoms reported a higher number of comorbidities and lower scores indicating worse general, mental and physical HRQOL compared to patients without depressive symptoms. We found no significant associations between pre-diagnosis depressive symptoms and poor mental HRQOL with all-cause or cancer-specific survival among older MM patients included in our study. Although not directly impacting survival, the high burden of depressive symptoms and poor mental health status among older patients with MM deserves clinical attention.
Depression and depressive symptoms may impact patients’ cancer treatment choices and could also lead to suboptimal treatment outcomes [9, 10]. The association between depression and cancer outcomes can be elucidated through the biobehavioral model for cancer progression [8, 7]. In brief, it is hypothesized that psychosocial stressors (i.e., depression) lead to higher resistance to cell death, compromised immunity, dysregulation of the autonomic nervous system, and inflammatory and oxidative stress, which in turn have detrimental effects on cancer progression [8]. Furthermore, depression is associated with longer hospitalizations, worse quality of life, poor medication adherence, and greater risk of suicide among cancer patients [8]. Among women with breast cancer, depression and use of antidepressants are also associated with non-adherence to adjuvant endocrine therapy [11, 12]. In the context of hematological cancers, pre-transplant depression is associated with lower all-cause survival and higher risk for acute graft rejection among allogenic stem-cell transplant recipients [10].
To our knowledge, all studies assessing depressive symptoms and poor mental HRQOL among MM patients were based on post-diagnosis surveys. A study of 305 MM patients with median age of 76 years reported poorer all-cause survival in patients with higher post-diagnosis frailty, which included components of mental and physical health among other factors[33]. Another study of 3019 MM patients with mean age of 69 years found no difference in MCS between newly diagnosed MM patients and patients with no history of cancer[34].
As for other cancers, very few studies evaluated the impact of pre-diagnosis depressive symptoms and mental HRQOL. In one study of postmenopausal breast cancer patients (ages 50 to 79 years), a 35% higher risk of all-cause mortality among patients with depression defined by the presence of pre-diagnosis depressive symptoms or use of antidepressants prior to cancer diagnosis (HR 1.35, 95% CI: 1.02–1.78) [13]. This association with pre-diagnosis depressive symptoms was found to be even greater in women who with late-stage breast cancer for both risks of all-cause (HR 2.00, 95% CI: 1.13–3.56) and cancer-specific death (HR 2.42, 95% CI: 1.24–4.70). In our study we identified depressive symptoms based on a positive DIS screen. It is possible that the null association with mortality risk was observed due to differences in defining our exposure. In one study of 6290 lung cancer patients ages 65 year and older using the SEER-MHOS data resource [20], a 4% lower risk for all-cause death was reported with five-point increase in MCS (HR 0.96, 95% CI: 0.95–0.98). A similar trend indicating an inverse association between higher mental HRQOL and mortality in patients with cancer was observed with a 24% decreased hazards of death among patients that scored above MCS population average (MCS 50 or higher). Another SEER-MHOS study of older patients with lung cancer found no significant association between MCS and overall survival (5 point HR 1.05, 95% CI: 0.98–1.11) [19]. While both studies included older patients with characteristics fairly similar to our cohort, the latter study differed in its requiring patients to have responded to a survey both prior to and following cancer diagnosis.
This analysis has strengths, especially the extensive and robust population-based information included in SEER-MHOS data. We were able to account for important factors such as patient demographics and comorbidities than can impact depressive symptoms, mental HRQOL, and survival. Furthermore, the majority of previous studies assessed depressive symptoms and HRQOL after cancer diagnosis; and to our knowledge, this is the first study evaluating the impact of pre-diagnosis depressive symptoms and poor mental HRQOL on cancer outcomes among MM survivors.
Our study also has several limitations. Common to survey-based observational studies, variable response by certain patient characteristics and inability to draw causal inference are possible limitations. Consistent with previous studies, we addressed variable survey response by adjusting for several demographic and clinical patient characteristics [34]. While the SEER registries cover approximately 35% of the U.S. population, certain regions such as states of Florida and Minnesota are not represented [22]. At the same time, SEER data are limited by a lack of information on some cancer treatments documented in the registries. Further, Medicare Advantage enrollees may differ systematically from others, such as Medicare fee-for-service beneficiaries; however, studies indicate conflicting results [35, 36]. Results from this study are not necessarily generalizable to patients younger than 65 years and residual confounding by unmeasured covariates is possible. We lacked information on other important clinical information including type and duration of treatment, receipt of bone marrow transplantation, disease recurrence and progression, severity of depression, and whether or not patients were receiving depression treatment. Our study characterized depressive symptoms assessed up to 5 years prior to cancer diagnosis. Consistent with other studies, longer time between depression screening and cancer diagnosis may attenuate a possible association with cancer survival if one exists [13].
Our null findings could be explained by several factors. Our study may have been underpowered to differentiate differences by subgroups of recency. Also, protective factors such as resilience and posttraumatic growth have been linked to favorable psychological and treatment-related outcomes among cancer survivors [37]. As a result, those factors can ameliorate the impact of pre-diagnosis depressive symptoms and poor mental health on mortality.
As advances in the treatment of MM lead to greater survival years, additional research and efforts to alleviate the psychosocial burdens associated with cancer are equally necessary. Older patients have higher risk for social isolation, which can be detrimental to their wellbeing [38]. Furthermore, the diagnosis of cancer and aggressive cancer treatment can further exacerbate the burdens of depression and poor mental health in this populations [11, 21]. After cancer treatment, psychosocial problems such as depression can hinder the ability of cancer survivors to successfully return to their work and social life [39]. Our findings highlight the high prevalence of pre-existing depressive symptoms and poor mental HRQOL among older patients diagnosed with MM. Larger studies of both younger and older MM patients treated in the novel agent era may help inform the evidence base for universal depression and HRQOL screening in the regular care of MM.
Conclusion
Depressive symptoms and mental HRQOL assessed prior to cancer diagnosis did not significantly impact survival of older patients with multiple myeloma. Additional research is essential to understand the high prevalence of pre-diagnosis depressive symptoms and poor mental HRQOL and explore their impact on future risks of major depressive disorder, treatment acceptability and clinical decision making following MM diagnosis. Lastly, prospective studies and clinical trials should determine whether life-prolonging novel therapies also improve mental HRQOL.
Supplementary Material
Acknowledgments
This study used data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare Health Outcomes Survey (MHOS) linked data resource. The authors acknowledge the efforts of the National Cancer Institute; the Centers for Medicare and Medicaid Services; MHOS; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-MHOS database. The National Cancer Institute provided suggested edits and approval of the manuscript before final journal submission.
Funding: Dr. Patel received consultancy (Celgene, Janssen) and honoraria (Celgene, Janssen, Amgen). Dr. Calip was supported by the National Institutes of Health, National Center for Advancing Translational Sciences through grant number KL2TR002002 and the National Heart, Lung and Blood Institute through grant number R21HL140531. Dr. Chiu was supported by the National Cancer Institute through grant number R01CA223662. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors have no competing interests to declare.
Compliance with Ethical Standards
Ethical approval: The data used in the present study were de- identified and compliant with the Health Insurance Portability and Accountability Act; this study was determined to be exempt by the Institutional Review Board of the University of Illinois at Chicago.
Informed consent: The Institutional Review Board of the University of Illinois at Chicago determined this study to be exempt from obtaining informed consent from individual participants.
Data Availability: The authors have full control of all primary data. The data that support the findings of this study are available from the SEER-Medicare Health Outcomes Survey Data Resource. Restrictions apply to the availability of these data, which were used under license for this study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Stat Facts: Myeloma. National Cancer Institute. Bethesda, MD: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed March 28th, 2019. [Google Scholar]
- 4.Bell JR. Underdiagnosis of depression in primary care: by accident or design? JAMA. 1997;277(18):1433. [PubMed] [Google Scholar]
- 5.Serby M, Yu M. Overview: depression in the elderly. Mt Sinai J Med. 2003;70(1):38–44. [PubMed] [Google Scholar]
- 6.Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand. 2006;113(5):372–87. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 7.Lutgendorf SK, Andersen BL. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. Am Psychol. 2015;70(2):186–97. doi: 10.1037/a0035730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Buscariollo DL, Cronin AM, Borstelmann NA, Punglia RS. Impact of pre-diagnosis depressive symptoms and health-related quality of life on treatment choice for ductal carcinoma in situ and stage I breast cancer in older women. Breast Cancer Res Treat. 2019;173(3):709–17. doi: 10.1007/s10549-018-5006-5. [DOI] [PubMed] [Google Scholar]
- 10.El-Jawahri A, Chen YB, Brazauskas R, He N, Lee SJ, Knight JM et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer. 2017;123(10):1828–38. doi: 10.1002/cncr.30546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30(2):112–26. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Calip GS, Xing S, Jun DH, Lee WJ, Hoskins KF, Ko NY. Polypharmacy and Adherence to Adjuvant Endocrine Therapy for Breast Cancer. J Oncol Pract. 2017;13(5):e451–e62. doi: 10.1200/JOP.2016.018317. [DOI] [PubMed] [Google Scholar]
- 13.Liang X, Margolis KL, Hendryx M, Reeves K, Wassertheil-Smoller S, Weitlauf J et al. Effect of depression before breast cancer diagnosis on mortality among postmenopausal women. Cancer. 2017;123(16):3107–15. doi: 10.1002/cncr.30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mols F, Husson O, Roukema JA, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. J Cancer Surviv. 2013;7(3):484–92. doi: 10.1007/s11764-013-0286-6. [DOI] [PubMed] [Google Scholar]
- 15.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11):1797–810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22):5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 17.Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L et al. Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;30(23):2885–90. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doll KM, Pinheiro LC, Reeve BB. Pre-diagnosis health-related quality of life, surgery, and survival in women with advanced epithelial ovarian cancer: A SEER-MHOS study. Gynecol Oncol. 2017;144(2):348–53. doi: 10.1016/j.ygyno.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinheiro LC, Reeve BB. Investigating the prognostic ability of health-related quality of life on survival: a prospective cohort study of adults with lung cancer. Support Care Cancer. 2018;26(11):3925–32. doi: 10.1007/s00520-018-4265-3. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro LC, Zagar TM, Reeve BB. The prognostic value of pre-diagnosis health-related quality of life on survival: a prospective cohort study of older Americans with lung cancer. Qual Life Res. 2017;26(7):1703–12. doi: 10.1007/s11136-017-1515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeve BB, Potosky AL, Smith AW, Han PK, Hays RD, Davis WW et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101(12):860–8. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, Clauser SB. Overview of the SEER--Medicare Health Outcomes Survey linked dataset. Health Care Financ Rev. 2008;29(4):5–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Overview of the SEER Program. https://seer.cancer.gov/about/overview.html. Accessed March 28th, 2019.
- 24.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 25.SEER-MHOS Response Rates. 2018. https://healthcaredelivery.cancer.gov/seer-mhos/aboutdata/table.response.rates.html. Accessed March 28th, 2019.
- 26.Clark CJ, Fino NF, Liang JH, Hiller D, Bohl J. Depressive symptoms in older long-term colorectal cancer survivors: a population-based analysis using the SEER-Medicare healthcare outcomes survey. Support Care Cancer. 2016;24(9):3907–14. doi: 10.1007/s00520-016-3227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White AJ, Reeve BB, Chen RC, Stover AM, Irwin DE. Coexistence of urinary incontinence and major depressive disorder with health-related quality of life in older Americans with and without cancer. J Cancer Surviv. 2014;8(3):497–507. doi: 10.1007/s11764-014-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rost K, Burnam MA, Smith GR. Development of screeners for depressive disorders and substance disorder history. Med Care. 1993;31(3):189–200. [DOI] [PubMed] [Google Scholar]
- 29.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12(7):439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selim AJ, Rogers W, Fleishman JA, Qian SX, Fincke BG, Rothendler JA et al. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res. 2009;18(1):43–52. doi: 10.1007/s11136-008-9418-2. [DOI] [PubMed] [Google Scholar]
- 31.Fleishman JA, Selim AJ, Kazis LE. Deriving SF-12v2 physical and mental health summary scores: a comparison of different scoring algorithms. Qual Life Res. 2010;19(2):231–41. doi: 10.1007/s11136-009-9582-z. [DOI] [PubMed] [Google Scholar]
- 32.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48(6 Suppl):S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 33.Mian HS, Wildes TM, Fiala MA. Development of a Medicare Health Outcomes Survey Deficit-Accumulation Frailty Index and Its Application to Older Patients With Newly Diagnosed Multiple Myeloma. 2018(2):1–13. doi: 10.1200/cci.18.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD. Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer. 2015;121(5):758–65. doi: 10.1002/cncr.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martino SC, Elliott MN, Haviland AM, Saliba D, Burkhart Q, Kanouse DE. Comparing the Health Care Experiences of Medicare Beneficiaries with and without Depressive Symptoms in Medicare Managed Care versus Fee-for-Service. Health Serv Res. 2016;51(3):1002–20. doi: 10.1111/1475-6773.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pourat N, Kagawa-Singer M, Wallace SP. Are managed care Medicare beneficiaries with chronic conditions satisfied with their care? J Aging Health. 2006;18(1):70–90. doi: 10.1177/0898264305280997. [DOI] [PubMed] [Google Scholar]
- 37.Seiler A, Jenewein J. Resilience in Cancer Patients. Front Psychiatry. 2019;10:208. doi: 10.3389/fpsyt.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickens AP, Richards SH, Greaves CJ, Campbell JL. Interventions targeting social isolation in older people: a systematic review. BMC Public Health. 2011;11:647. doi: 10.1186/1471-2458-11-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duijts SF, van Egmond MP, Spelten E, van Muijen P, Anema JR, van der Beek AJ. Physical and psychosocial problems in cancer survivors beyond return to work: a systematic review. Psychooncology. 2014;23(5):481–92. doi: 10.1002/pon.3467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.