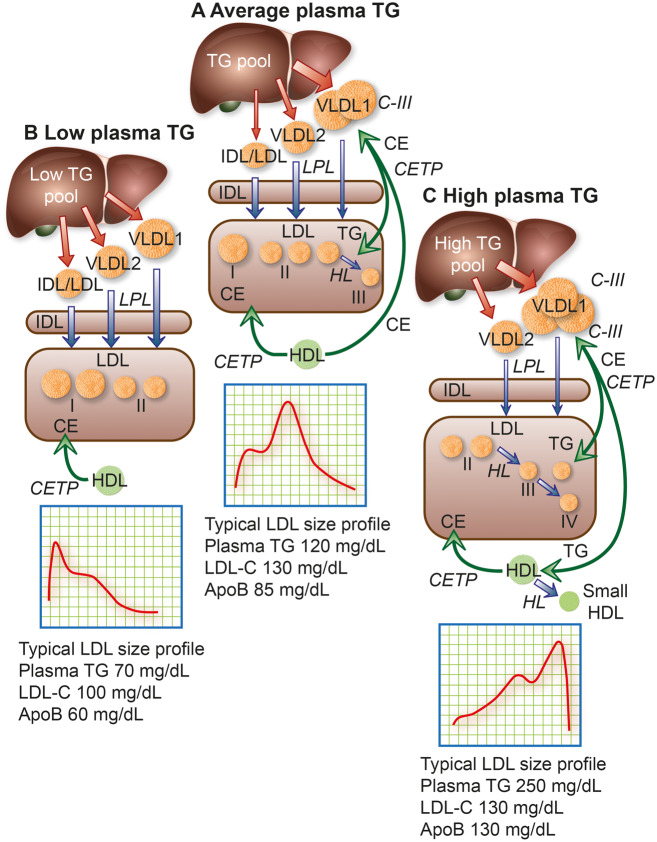
Figure 2.
Model of the metabolic interrelationships between low-density lipoprotein (LDL) subfractions and their hepatic precursors. The liver produces apolipoprotein (apo)B100-containing particles ranging in size from large triglyceride (TG)-rich very low-density lipoprotein (VLDL) 1, through small VLDL2 and intermediate-density lipoprotein (IDL) to LDL.74 The hepatic TG content (TG pool) affects the profile of the secreted particles.99 Secreted VLDL undergoes lipolysis and remodelling to form remnants/IDL; LDL is then formed via the actions of lipoprotein lipase (LPL), hepatic lipase (HL), and cholesteryl ester transfer protein (CETP). (A) In people with population average TG levels, about half the lipolytic remnants (which correspond to IDL based on density and size) in this pathway are cleared relatively efficiently and the remainder are converted mainly to LDL-II, which has higher LDL receptor affinity and shorter residence time than the LDL arising from VLDL1.74,79,82,83 The composition of IDL-derived LDL is modulated both by CETP-mediated transfer of cholesteryl esters (CE) from high-density lipoprotein (HDL) and by CETP-mediated transfer of TG from VLDL and their remnants.102,106 (B) In individuals with low plasma TG, LDL-I and -II predominate. Clearance of these lipoproteins is rapid and LDL-cholesterol (LDL-C) and apoB concentrations are low. (C) Individuals with elevated plasma TG levels overproduce VLDL1 and have reduced lipolysis rates due in part to inhibition of LPL activity by their abundant content of apoC-III, an LPL inhibitor. Very low-density lipoprotein 1 remodelling gives rise to remnants within the VLDL size range that are enriched in apoE; such circulating remnants can be removed by several mechanisms, primarily in the liver, including the LDL receptor-related protein, heparan sulfate proteoglycans, and LDL receptor.107–109 Hepatic clearance of VLDL1-derived remnant particles may, however, be slowed by enrichment with apoC-III.78 Very low-density lipoprotein 1 and VLDL2 are targeted by CETP, which exchanges core CE in LDL for TG in both VLDL1 and VLDL2. Hydrolysis of TG by HL action then shrinks LDL particles to preferentially form small, dense LDL-III in moderate hypertriglyceridaemia, or even smaller LDL-IV in severe hypertriglyceridaemia; such small dense LDL exhibit attenuated binding affinity for the LDL receptor, resulting in prolonged plasma residence (Box 2). Together, this constellation of lipoprotein changes, originating in increased levels of large VLDL1 and small dense LDL, represents a lipid phenotype designated atherogenic dyslipidaemia,6–8,74,75,79–81,110 a key feature of metabolic syndrome and Type 2 diabetes.6–8,78–80 Typical LDL subfraction patterns are indicated together with relevant plasma lipid and apoB levels. Note that when small dense LDL is abundant, apoB is elevated more than LDL-C. The width of the red arrows reflects the quantity of apoB/particle production and release from the liver, while the width of the blue arrows depicts relative lipolytic efficiency.