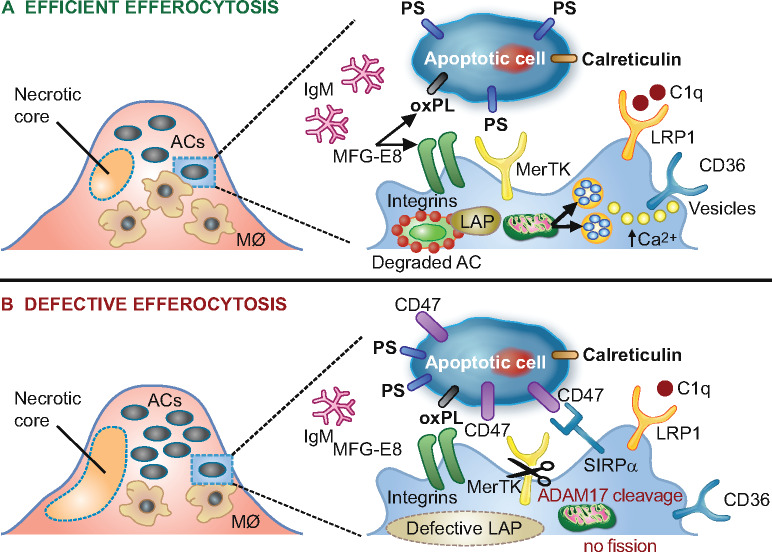
Figure 4.
Schematic representation of processes involved in lesional efferocytosis. (A) Externalized ‘eat me’ signals including phosphatidylserine (PS), calreticulin, and oxidized phospholipids (oxPL) are recognized by their respective receptors, Mer tyrosine kinase (MerTK), low-density lipoprotein-receptor-related protein 1 (LRP1), as well as integrin αvβ3 and CD36 on macrophages; such recognition is facilitated either directly or mediated by bridging molecules such as growth arrest-specific 6 for PS, complement protein C1q for calreticulin and milk fat globule-epidermal growth factor 8 (MFG-E8) for oxPL. Calcium-dependent vesicular trafficking events driven by mitochondrial fission and LC3-associated phagocytosis (LAP) promote phagolysosomal fusion and the hydrolytic degradation of apoptotic cells. Simultaneously, natural immunoglobulin (Ig)M antibodies with reactivity towards oxidation-specific epitopes further enhance the efficient clearance of dying cells via complement receptors. (B) In advanced atherosclerosis, one or more of these mechanisms are dysfunctional and can lead to defective efferocytosis, propagating non-resolving inflammation and plaque necrosis. Additional processes contributing to impaired efferocytosis include ADAM-17-mediated cleavage of MerTK as well as the inappropriate expression of the ‘don’t eat me’ signal CD47 on apoptotic cell surfaces. ACs, apoptotic cells.