Table 1.
Reaction optimization.a
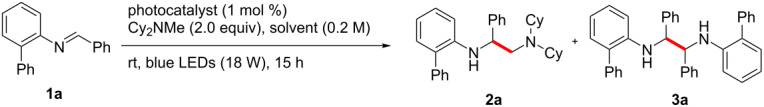 | |||||
| entry | photocatalyst | variation | solvent | yield (%)b | |
| 2a | 3a | ||||
| 1 | [Ru(bpy)3]Cl2⋅6H2O | – | CH3CN | 12 | 0 |
| 2 | [Ru(bpz)3](PF6)2 | – | CH3CN | 0 | 0 |
| 3 | Ir(dFppy)3 | – | CH3CN | 0 | 0 |
| 4 | Ir(ppy)3 | – | CH3CN | 3 | 25 |
| 5 | [Ir(dF(CF3)ppy)2(dtbpy)]PF6 | – | CH3CN | 38 | 5 |
| 6 | [Ir(dtbbpy)(ppy)2]PF6 | – | CH3CN | 60 | 11 |
| 7 | TTPP | – | CH3CN | 0 | 0 |
| 8 | crystal violet | – | CH3CN | 0 | 0 |
| 9 | eosin-Y | – | CH3CN | 38 | 0 |
| 10 | [Ir(dtbbpy)(ppy)2]PF6 | Cy2NMe (1 equiv) | CH3CN | 14 | 55 |
| 11 | [Ir(dtbbpy)(ppy)2]PF6 | no Cy2NMe | CH3CN | 0 | trace |
| 12 | no catalyst | – | CH3CN | 0 | 0 |
| 13 | [Ir(dtbbpy)(ppy)2]PF6 | no light | CH3CN | 0 | 0 |
| 14 | [Ir(dtbbpy)(ppy)2]PF6 | – | DCM | 0 | 16 |
| 15 | [Ir(dtbbpy)(ppy)2]PF6 | – | DMF | 72 (71) | 7 |
| 16 | [Ir(dtbbpy)(ppy)2]PF6 | – | DCE | 22 | 11 |
| 17 | [Ir(dtbbpy)(ppy)2]PF6 | – | DMSO | 37 | 6 |
| 18 | [Ir(dtbbpy)(ppy)2]PF6 | – | dioxane | 50 | 13 |
| 19 | [Ir(dtbbpy)(ppy)2]PF6 | – | TFE | 0 | 20 |
| 20 | [Ir(dtbbpy)(ppy)2]PF6 | – | acetone | 22 | trace |
| 21 | [Ir(dtbbpy)(ppy)2]PF6 | – | EtOAc | 36 | 8 |
| 22 | [Ir(dtbbpy)(ppy)2]PF6 | – | CH3OH | 4 | 75 (75) |
| 23 | [Ir(dtbbpy)(ppy)2]PF6 | – | DMF/CH3OH (1:1) | 35 | 29 |
| 24 | [Ir(dtbbpy)(ppy)2]PF6 | – | DMF/H2O (1:1) | 25 | 10 |
| 25 | [Ir(dtbbpy)(ppy)2]PF6 | – | CH3CN/H2O (1:1) | 15 | 14 |
aReaction conditions: 1a (0.1 mmol), under argon atmosphere. bThe yields were determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as the internal standard, and the isolated yields are mentioned in parentheses.
