Figure 2.
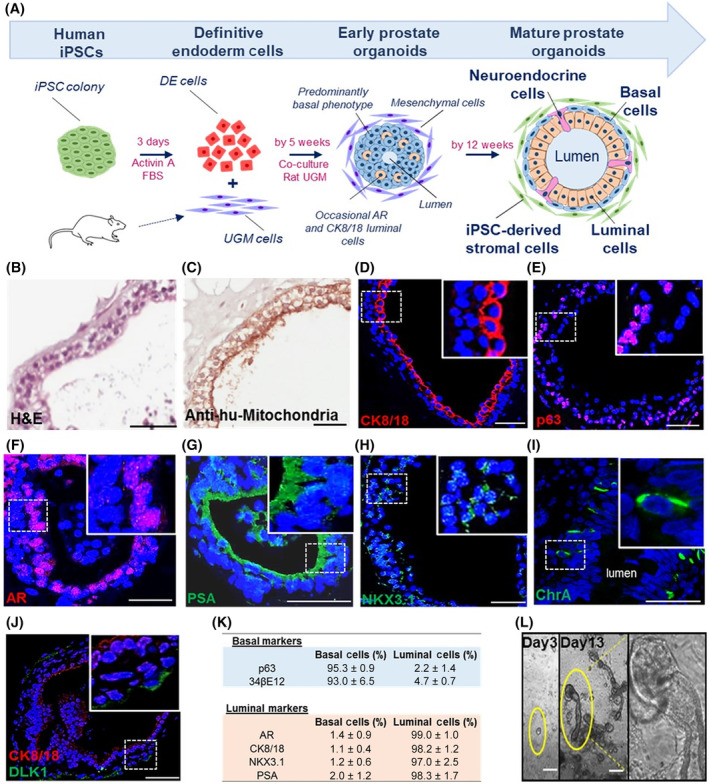
In vitro generation of human prostate organoids. A, Schema outlining the differentiation process (n = 3 induced pluripotent stem cell [iPSC] clones). Briefly, human prostate‐derived iPSCs were differentiated to definitive endoderm (DE) cells using activin A and fetal bovine serum (FBS) over 3 days. Resultant cells were subsequently cocultured with rat urogenital sinus mesenchyme (UGM). At 5 weeks, early prostate organoids were observed having a predominantly basal phenotype while occasionally contained small lumens and expressed luminal markers. Multilayered organoids with large lumens demonstrating classical prostate‐like histology by forming an outer basal and inner luminal layer were observed by 12 weeks. B, Histology of organoids resembled prostate glands. C, Epithelium was identified as human by antihuman mitochondria staining. D, CK8/18 on the cell surface confirmed luminal cells. E, Nuclear p63 confirmed basal cells. F‐H, Androgen receptor (AR), prostate‐specific antigen, and NKX3.1 expression by luminal cells confirmed terminal differentiation. I, Neuroendocrine cells were identified by ChrA expression (0.64 ± 0.21% respectively, n = 7600 cells, n = 12 organoids). J, A subpopulation of basal cells expressed the somatic stem cell marker DLK1 (3.0 ± 1.3%, n = 650 cells, n = 3 experiments). Scale bar = 50 μm. K, Reproducible expression of differentiation markers in mature prostate organoids is shown (n = 183 organoids [164 ± 33 cells/organoid], n = 3 separate experiments; see description of calculations in Materials and Methods). L, Following passage, early clone formation from 1 to 2 cells was observed on day 3, while by day 13 clear canalization was noted of tubular structures associated with dense bud tips reminiscent of tubular branching patterns seen in organogenesis. Scale bar = 25 μm
