Abstract
Patients with severe mental illness are more susceptible to infections for a variety of reasons, some associated with the underlying disease and some due to environmental factors including housing insecurity, smoking, poor access to healthcare, and medications used to treat these disorders. This increased susceptibility to respiratory infections may contribute to risk of COVID-19 infection in patients with severe mental illness or those in inpatient settings. Atypical antipsychotic (AA) medications are FDA approved to treat symptoms associated with schizophrenia, bipolar disorder, depression and irritability associated with autism. Our team and others have shown that AA may have anti-inflammatory properties that may contribute to their efficacy in the treatment of mental health disorders. Additionally, AA are widely prescribed off-label for diverse indications to non-psychotic patients including older adults, who are also at increased risk for COVID-19 complications and mortality. The aim of this study was to determine if AA medications such as risperidone (RIS) alter the ability to mount an appropriate response to an acute inflammatory or adaptive immune challenge using a preclinical model. Short-term treatment of healthy mice with a dose of RIS that achieves plasma concentrations within the low clinical range resulted in disrupted response to an inflammatory (LPS) challenge compared to vehicle controls. Furthermore, RIS also prevented treated animals from mounting an antibody response following vaccination with Pneumovax23®. These data indicate that short-to intermediate-term exposure to clinically relevant levels of RIS dysregulate innate and adaptive immune responses, which may affect susceptibility to respiratory infections, including COVID-19.
Keywords: Antipsychotic, Adaptive immunity, Inflammation, Pneumonia, Infection, Risperidone, Cytokine
Highlights
-
•
Risperidone caused a highly dysregulated immune response in healthy mice.
-
•
Short term, low-dose risperidone therapy altered cytokine response to LPS challenge.
-
•
Risperidone caused failure to seroconvert following vaccination with Pneumovax23®.
-
•
Antipsychotic-induced immune dysfunction has serious implications for older adults.
-
•
Antipsychotic immune-dysregulation has implications for COVID-19 vulnerable patients.
1. Introduction
The novel betacoronavirus SARS-CoV-2 emerged in late 2019, causing an acute respiratory distress syndrome (ARDS) known as COVID-19. The risk of complications and mortality increases with age and significant comorbidities (Guan et al., 2020), such as patients residing in long-term care facilities (LTCFs). Poor and vulnerable patient populations are disproportionately impacted by disasters such as the COVID-19 pandemic. Among those, patients with severe mental illness may be especially vulnerable due to a variety of factors including those relating to housing (housing insecurity or increased risk of exposure in residential treatment centers or prison settings) effects of underlying disease, side effects of medications, poor access to healthcare and lifestyle factors such as smoking (Druss, 2020; Akiyama et al., 2020). As of May 11, 2020, nearly 4.2 million patients have been infected with SARS-CoV-2 world-wide, many of whom are associated with LTCFs. Respiratory tract infections (RTIs) are a significant cause of death for patients with schizophrenia (Olfson et al., 2015; Haga et al., 2018). Given the absence of effective treatment for COVID-19, factors that could exacerbate immune dysfunction in vulnerable patient populations require close scrutiny.
Atypical antipsychotic (AA) drugs are FDA-approved first line therapy for the treatment of schizophrenia, bipolar disorder, severe depression and irritability associated with autism. AA are prescribed off-label for diverse indications to non-psychotic patients including older adults, and are among the most highly prescribed medications across age ranges. In fact, up to one third of all LTCF patients are administered AA to control agitation and aggression associated with dementia, despite carrying a black box warning for sudden death in these patients (Reus et al., 2016), and a small number of clinical reports associate AA with an increased risk of infection (most notably urinary; van Strien et al., 2018) and respiratory tract (Tolppanen et al., 2016) infections.
We previously reported global immunosuppression, myeloid dysplasia in the bone marrow, and thymic involution by the AA risperidone (RIS), and disruption of mitochondrial function and the cardio-immune axis by RIS and olanzapine in healthy individuals using a preclinical model (May et al., 2019; Beauchemin et al., 2020; Rostama et al., 2020). However, the evidence for AA-associated immune dysregulation during acute or adaptive immune challenge is lacking. Therefore, the overarching goal of this work was to determine the effect of short-to moderate-term AA exposure on the ability to respond to immune challenge.
2. Materials and methods
2.1. Animal care and use
The Institutional Animal Care and Use Committee (IACUC) at the University of New England approved all animal studies.
The preclinical model used for this study employed 8 week old male C57BL/6J mice, fed standard mouse chow (18% protein rodent chow; Envigo) and treated by oral gavage once daily with vehicle (VEH; 0.1% acetic acid) or a low dose of RIS for 14 days (Sigma; 1 mg/kg). This drug dose was chosen based upon preliminary PK studies conducted in our laboratory (Motyl et al., 2017) which reflect plasma drug exposure consistent with what is observed clinically (Mauri et al., 2014). At the end of each treatment period, mice were sacrificed by CO2 asphyxiation and plasma (EDTA) was collected for analysis.
2.2. Drug exposure
Plasma concentrations of RIS and the active metabolite paliperidone (PAL) were measured at the time of necropsy to ensure proper dosing and drug metabolism. Concentrations of RIS and PAL were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis as previously reported (Motyl et al., 2017). Briefly, RIS and PAL were extracted from both plasma and bone marrow via protein precipitation with acetonitrile. Separation was accomplished using a Waters XBridge C18 analytical column (3.0 × 50 mm, 3.5 μm). Mobile phase consisted of 0.1% formic acid in purified water (A) and 0.1% formic acid in acetonitrile (B). The flow rate was 0.4 mL/min, and heated to 60 °C. Gradient elution was employed, with initial conditions 95% A and 5% B. Solvent composition was held at the initial conditions for 1.0 min, and then was ramped over the following 1.5 min to 95% B. Composition was maintained at 95% B for 1 min. RIS and PAL were detected via an Agilent (Waldbronn, Germany) 6460 triple quadrupole mass spectrometer operated in positive ion MRM mode. The following transitions were monitored: RIS (411.2 → 191.0) and PAL (427.2 → 207.0).
2.3. Acute inflammatory challenge study
To assess the impact of RIS on the acute inflammatory response, RIS and DVEH mice were given an intraperitoneal injection of either a sub lethal dose of lipopolysaccharide (LPS, 500 ng/kg, diluted in PBS; 14) or vehicle (LVEH, PBS) after 14 days of treatment (Copeland et al., 2005; Supplemental Table S1). Two hours post-injection, mice were sacrificed by CO2 asphyxiation and blood was collected for subsequent analysis. Circulating cytokine, chemokine, and other immune factor levels were quantified using the Proteome Profiler Cytokine Array (R&D BioSystems) per the manufacturer’s instructions. Arrays were imaged using a FluorChem Q instrument and quantified using ImageQuant v. 8.2. Each duplicate array spot representing a unique immune marker was normalized to each of three reference spots per array (N = 3 measurements per spot). Normalized measurements were averaged for each spot, and the duplicate spots for each feature were then averaged to generate a total measure for each immune marker. Unique arrays were run for each animal. Statistical significance signaling a change in response to inflammatory challenge was determined for each immune factor by two-tailed t-test between DVEH/LVEH and DVEH/LPS groups, and RIS/LVEH and RIS/LPS groups. Factors with the same effect observed between DVEH and RIS group relative to their respective controls were considered unaffected, whereas those with a different effect were considered dysregulated. Measured factors are listed in Supplementary Table S2.
2.4. Immunological function pathway analysis
Entries for all measured cytokines were accessed from the Kyoto Encyclopedia of Genes and Genomes (KEGG) Database (Kanehisa et al., 2017). Pathways for each measured factor involved in immune function (N = 24 pathways) or response to respiratory tract infection (N = 5 pathways) were pulled and analyzed. Pathways involving measured features that are not linked to immune function or infectious disease responses were not included in the analysis. Immune marker levels of DVEH-treated mice following exposure to LPS relative to levels of DVEH-treated mice following exposure to LVEH were considered a normal, functional response (regardless of change). The numbers of immune markers in RIS-treated mice that had a normal response following exposure to LPS relative to RIS-treated, LVEH-exposed mice (i.e., that of DVEH/LPS mice) were tabulated. RIS-treated mice that had a significantly different response from DVEH/LPS mice were also tabulated. Heat maps reflecting the relative functionality and dysregulation of each KEGG pathway analyzed were generated. Each immune factor present at abnormal levels following LPS challenge during RIS treatment in a pathway was allotted proportional wavelength in their assigned color on a scale from 55 nm to 255 nm (dysregulated factors = red; factors at normal levels = green). Multiple factors contributing to either functionality or dysregulation contribute additively to color intensity.
2.5. Adaptive immune challenge study
To assess the impact of RIS on adaptive immunity, RIS and DVEH mice were immunized by intramuscular injection with either Pneumovax23® (VAX) or vehicle (VVEH, sterile water) after 5 days of treatment with RIS or DVEH (Table S1). Oral administration of RIS or DVEH continued through day 14, when mice were sacrificed as described above. Anti-pneumococcal IgG antibodies were quantified by direct enzyme-linked immunosorbent assay (myBioSource) per manufacturer’s instructions. Intensity of the chromogenic substrate 3,3′,5,5′-Tetramethylbenzidine plus 2N H2SO4 stop solution was detected using an M5 SpectraMax plate reader (optical density ƛ = 450 nm). Statistical significance indicating a change in capacity for antibody production in response to vaccination was determined by one-tailed t-test between DVEH/VAX and RIS/VAX groups. Individuals were defined as “responders” or “non-responders” by χ2 analysis between both DVEH/VVEH and DVEH/VAX groups and RIS/VVEH and RIS/VAX groups.
3. Results
3.1. Animal health
The dose of drug selected for these studies results in total plasma drug concentrations that fall in the low end of the clinical range and causes no significant change in feeding, body weight or general behavior as previously published (Beauchemin et al., 2020; Motyl et al., 2012, 2015, 2017; May et al., 2019). In all treatment cohorts, animals appeared healthy and gained weight, as expected.
In the acute inflammatory challenge study, DVEH/LVEH animals (n = 6) weighed 25.1±1.3 on day 1 and 26.4±0.73 at the culmination of the study. DVEH/LPS animals (n = 6) weighed 24.0±1.14 g on day 1 and 25.8±1.16 g at the end of the study. RIS/LVEH animals (n = 6) weighed 25.8±1.27 g on day 1 and 27.4±1.18 on the final day. RIS/LPS animals averaged 24.1±0.59 g BW on day 1 and 25.6±0.61 g on day last. The dose of LPS employed in this study is sub-lethal, and as expected, all animals survived LPS treatment. Plasma concentrations of RIS and PAL averaged 11.3±1.7 and 47.9±6.3 nM in RIS treated animals. No drug concentrations were detectable in samples from VEH treated animals.
In the adaptive immune challenge study, average body weights on day 1 were 24.4±0.59, 24.2±1.17, 23.6±0.76 and 23.9±0.33 g for DVEH/VVEH, DVEH/VAX, RIS/VVEH and RIS/VAX animals, respectively (n = 6 per treatment). Mean body weights on day 15 were 26.1±0.06, 26.2±1.05, 25.1±0.80 and 25.7±0.50 g for DVEH/VVEH, DVEH/VAX, RIS/VVEH and RIS/VAX animals (n = 6 per treatment). Plasma concentrations of RIS and PAL averaged 12.6±2.9 and 47.1±5.7 nM in RIS treated animals. No drug concentrations were detectable in samples from VEH treated animals.
3.2. Acute inflammatory biomarkers
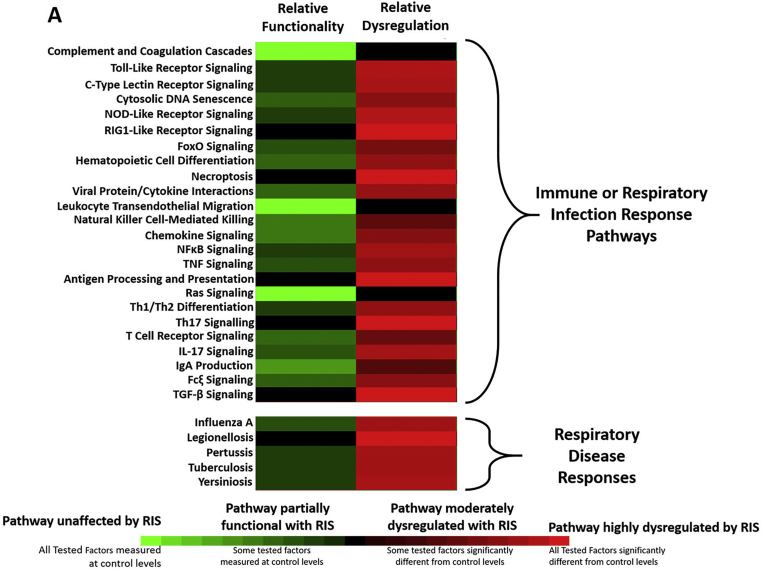
Low-dose RIS treatment of previously healthy animals led to significant dysregulation of the acute inflammatory response upon challenge. Only 3 pathways (complement and coagulation cascades, Ras signaling pathway, and leukocyte transendothelial migration pathway) of 24 pathways were completely functional and all respiratory tract infection response pathways were at least partially dysregulated (Fig. 1). Of the thirty-one cytokines assessed, nineteen were significantly altered/dysregulated in RIS/LPS individuals relative to DVEH/LPS individuals, including the measurable clinical markers TNF-α and IL-6 (Supplemental Table S2; Supplemental Figure S1).
Fig. 1.
Innate Immunity is Confounded by the AA Risperidone. The robustness of innate immunity was measured by introducing an acute inflammatory challenge, LPS, and measuring 31 circulating immune factors integral to several immune function and respiratory disease response pathways in the KEGG database. The number of factors in each pathway whose responses were dysregulated (red) or unaffected (green) by RIS relative to DVEH controls were tabulated for each pathway. Only the complement and coagulation cascade, Ras signaling pathway, and leukocyte transendothelial migration pathways were unaffected by RIS, and all others showed some degree of dysregulation. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Antibody response to immunization
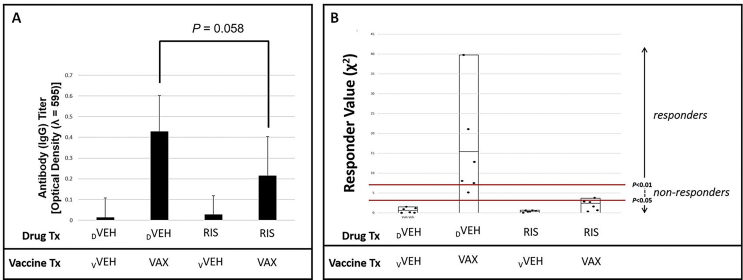
Overall mean anti-pneumococcal antigen IgG response values for animals treated with DVEH that received VAX injections generated were significantly (P < 0.0001) greater than DVEH-treated mice receiving VVEH, and significantly (P < 0.05) greater than mice treated with RIS prior to vaccination (Fig. 2a). Measured as individual responders, animals treated with RIS were significantly unable (P < 0.01) or less likely (P < 0.05) to mount an antibody response following immunization with Pneumovax23® relative to DVEH treated controls (Fig. 2b).
Fig. 2.
Adaptive Immunity is Confounded by the AA Risperidone. Functionality of adaptive immune responses was assessed by measuring antibody responses following vaccination. To determine the ability to mount an immune response to antigen, RIS and DVEH mice were administered Pneumovax23 (VAX) or vehicle (vVEH, sterile water). Antibody titers against pneumococcal antigen were significantly diminished in RIS-treated individuals (panel A). Individuals’ responder values (χ2) are shown, and black bars represent the group mean (panel B). No RIS-treated individuals were vaccine responders at the P < 0.01 level, and only one was a responder at the P < 0.05 level (red bars). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The data presented in this report, which were generated using a dose of RIS that results in low, clinically relevant plasma drug concentrations, are consistent with the hypothesis that the AA drug RIS is highly dysregulatory to the immune response during both acute and adaptive responses. The affinity of AA drugs for diverse G protein-coupled receptors (GPCRs) including dopaminergic, serotoninergic, alpha-adrenergic, histaminergic and muscarinic receptors and drug distribution to the bone marrow compartment suggest a mechanistic basis for immune cell effects as well as the neuronal cell effects for which they were designed (Vidal and Pacheco, 2020). Our previous preclinical studies demonstrated profound impact of AA administration on inflammatory and adaptive immune factors in multiple tissues (blood, heart, liver) in the absence of substantial immune challenge (May et al., 2019; Beauchemin et al., 2020; Rostama et al., 2020). The COVID-19 pandemic led to an urgent need to evaluate the impact of AA drugs on innate and adaptive immune challenges in a controlled, preclinical setting.
In addition to high mortality rates associated with off-label AA use in LTCF patients, AA drugs have also been associated with a generally elevated risk for death by infection in psychiatric patients aged 50–60 years living on their own (Li et al., 2018). This suggests that it is plausible to expect a higher risk of mortality from COVID-19 infection in patients using AA drugs in an outpatient setting (for diverse psychiatric indications) as well as those in in-patient behavioral health centers, LTCFs, and the prison system. Our findings showing inflammatory and adaptive immune process dysregulation by RIS are especially troubling given the emerging understanding of inflammatory sepsis preceding rapid death in COVID-19 patients. Recent reports have implicated Toll-like receptor 4 (TLR4)-mediated signaling in patient responses to COVID-19, making our observations of dysregulated response to the TLR4 agonist LPS highly relevant. Cytokines and chemokines that increase during normal TLR4 signaling responses include TNF-α, IL-6, IL-1β, IL-12, IL-8, CCL5, MIP1-α, MIP1-β, CxCL10, CxCL9, and CxCL11 (Kanehisa et al., 2017). Of the 8 evaluated in this study, 3 (TNF-α, IL-6, and CxCL10) were significantly diminished by RIS and another 2 (MIP1-β and CxCL11) were dysregulated. Cytokines and chemokines reported to be elevated in COVID-19 patients include TNF-α, IL-6, IFNγ, CxCL10, CCL7, IL-1ra, CCL2, IL-1 α/β, MIP1-α, MIP1-β, IL-4, IL-10, and G-CSF (Vaninov, 2020; Ye et al., 2020). Of the 10 evaluated in this study, RIS significantly diminished 4 (TNF-α, IL-6, IFNγ, CxCL10, and CCL2) and dysregulated 4 (IL-1ra, IL-1α, IL-4, and MIP1-β). Only IL-10 and G-CSF levels were not altered by RIS during challenge with TLR4 agonist LPS, indicating that COVID-19 responses may be highly impacted by this drug. Furthermore, failure of RIS-treated mice to respond to immunization against pneumococcal antigen is alarming given the increasing concern about secondary bacterial pneumonia, including pneumococcal pneumonia, during ARDS due to SARS-CoV-2 (Wu et al., 2020).
Taken together, our findings strongly indicate that consideration of AA use should be carefully weighed against the risk of adverse outcomes during infection in both outpatient and inpatient settings. Most notably, patients treated with AA medications in LTCFs, residential behavioral health treatment centers, group homes, or prisons must be carefully monitored during the COVID-19 pandemic.
Author contributions
Concept and Design: M. May and K. L. Houseknecht.
Acquisition, analysis and interpretation of data: M. Slitzky, B. Rostama, M. May, D. Barlow, K.L. Houseknecht.
Drafting of the manuscript: M. May and K.L. Houseknecht.
Critical revision of the manuscript: M. May, M. Slitzky, B. Rostama, D. Barlow, K.L. Houseknecht.
Statistical analysis: M. May.
Supervision: M. May and K.L. Houseknecht.
Funding/support
Research reported in this publication was supported by grants from the National Institutes of Health including NIDDK Award number DK095143 (Houseknecht). We are grateful to Ms. Denise Giuvelis (University of New England Behavioral and Genotyping Core) for technical support (core received financial support provided by the NIGMS Award P20GM103643 [Meng]). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of competing interest
None reported.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100097.
Contributor Information
Meghan May, Email: mmay3@une.edu.
Karen L. Houseknecht, Email: khouseknecht@une.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Akiyama M.J., Spaulding A.C., Rich J.D. Flattening the curve for incarcerated populations - covid-19 in jails and prisons. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin M., Geguchadze R., Guntur A.R. Exploring mechanisms of increased cardiovascular disease risk with antipsychotic medications: risperidone alters the cardiac proteomic signature in mice. Pharmacol. Res. 2020 Feb;152:104589. doi: 10.1016/j.phrs.2019.104589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland S., Warren H.S., Lowry S.F., Calvano S.E., Remick D. Inflammation and the Host Response to Injury Investigators. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 2005 Jan;12(1):60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druss B.G. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatr. 2020 doi: 10.1001/jamapsychiatry.2020.0894. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga T., Ito K., Sakashita K., Iguchi M., Ono M., Tatsumi K. Risk factors for pneumonia in patients with schizophrenia. Neuropsychopharmacol. Rep. 2018 Dec;38(4):204–209. doi: 10.1002/npr2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M.1, Furumichi M.2, Tanabe M.2, Sato Y.3, Morishima K.2. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ye X., Zhao Z., Gao P., Jiang Y. Overlooked fatal infectious diseases after long-term antipsychotic use in patients with psychiatric illness. Schizophr. Res. 2018;195:258–259. doi: 10.1016/j.schres.2017.09.033. [DOI] [PubMed] [Google Scholar]
- Mauri M.C., Paletta S., Maffini M., Colasanti A., Dragogna F., Di Pace C. Clinical pharmacology of typical antipsychotics: an update. EXCLI J. 2014;13:1163–1191. [PMC free article] [PubMed] [Google Scholar]
- May M., Beauchemin M., Vary C., Barlow D., Houseknecht K.L. The antipsychotic medication, risperidone, causes global immunosuppression in healthy mice. PloS One. 2019;14(6) doi: 10.1371/journal.pone.0218937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl K.J., Dick-de-Paula I., Maloney A.E., Lotinun S., Bornstein S., de Paula F.J. Trabecular bone loss after administration of the second-generation antipsychotic risperidone is independent of weight gain. Bone. 2012 Feb;50(2):490–498. doi: 10.1016/j.bone.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl K.J., DeMambro V.E., Barlow D., Olshan D., Nagano K., Baron R. Propranolol attenuates risperidone-induced trabecular bone loss in female mice. Endocrinology. 2015 Jul;156(7):2374–2383. doi: 10.1210/en.2015-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl K.J., Beauchemin M., Barlow D. A novel role for dopamine signaling in the pathogenesis of bone loss from the atypical antipsychotic drug risperidone in female mice. Bone. 2017 Oct;103:168–176. doi: 10.1016/j.bone.2017.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., Gerhard T., Huang C., Crystal S., Stroup T.S. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatr. 2015 Dec;72(12):1172–1181. doi: 10.1001/jamapsychiatry.2015.1737. [DOI] [PubMed] [Google Scholar]
- Reus V.I., Fochtmann L.J., Eyler A.E. The American psychiatric association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am. J. Psychiatr. 2016;173(5):543–546. doi: 10.1176/appi.ajp.2015.173501. [DOI] [PubMed] [Google Scholar]
- Rostama B., May M., Houseknecht K.L. Atypical antipsychotic medications disrupt the cardio-metabolic and cardio-immune axes. Intervent Cardiol. 2020;12(2):11–16. [Google Scholar]
- Tolppanen A.M., Koponen M., Tanskanen A. Antipsychotic use and risk of hospitalization or death due to pneumonia in persons with and those without alzheimer disease. Chest. 2016;150(6):1233–1241. doi: 10.1016/j.chest.2016.06.004. [DOI] [PubMed] [Google Scholar]
- van Strien A.M., Souverein P.C., Keijsers C.J.P.W., Heerdink E.R., Derijks H.J., van Marum R.J. Association between urinary tract infections and antipsychotic drug use in older adults. J. Clin. Psychopharmacol. 2018;38(4):296–301. doi: 10.1097/JCP.0000000000000895. [DOI] [PubMed] [Google Scholar]
- Vaninov N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020 May;20(5):277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal P.M., Pacheco R. The cross-talk between the dopaminergic and the immune system involved in schizophrenia. Front. Pharmacol. 2020 doi: 10.3389/fphar.2020.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-P., Fatima A., Highland K. Recognition and management of respiratory coinfection and secondary bacterial pneumonia in patients with COVID-19. Cleve. Clin. J. Med. 2020 May doi: 10.3949/ccjm.87a.ccc015. ccc015. [DOI] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine storm’ in COVID-19. J. Infect. 2020 Jun;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.