Fig. 5.
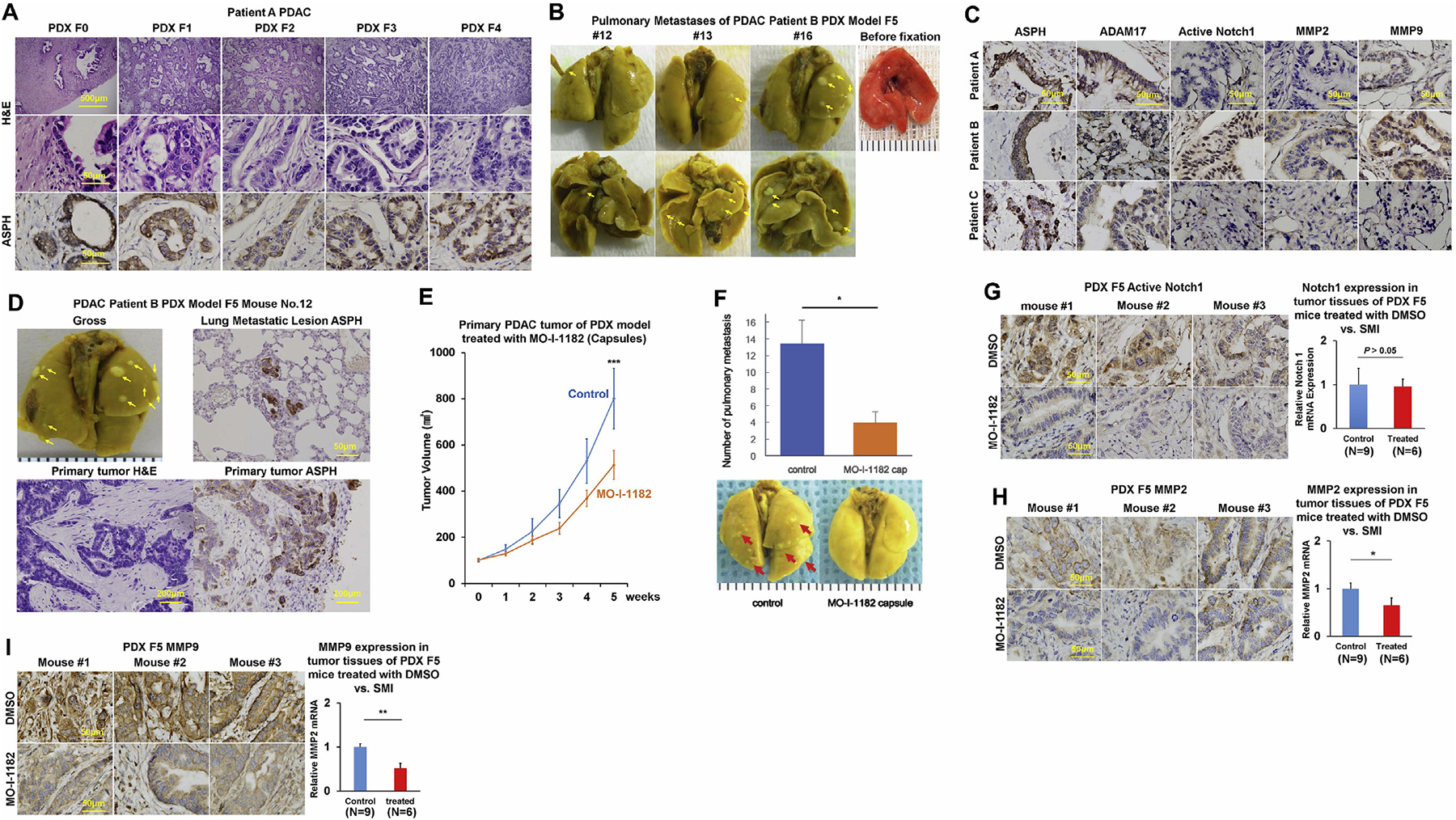
In vivo antitumor effects of a 3rd generation SMI (MO-I-1182) targeting ASPH enzymatic activity on PDX murine models of human PDAC.
*p < 0.05; **p < 0.01; ***p < 0.001.
(A) Histopathologic characteristics (H&E) of original tumors (F0) derived from a representative PDAC Patient A and xenografted tumors in representative mice of F1 through F4 generation PDX model.
(B) Pulmonary macro-metastases (arrows) in representative F5 PDX mice (n = 16) derived from Patient B. 100% (16/16) of the F5 generation PDX mice derived from Patient B had spontaneously developed pulmonary metastases.
(C) Expression profiling of ASPH-Notch components in resected primary PDAC tumor specimens derived from Patients A, B and C.
(D) Gross appearance of the involved lungs, histopathologic characteristics (H&E) and expression profiling of ASPH in transplanted primary tumors as well as pulmonary macro-metastases (arrows) in a representative mouse of F5 generation PDX model derived from PDAC Patient B.
(E) Transplanted primary tumor growth in mice of F5 generation PDX model in response to orally formulated SMI vs. DMSO control. It took 4–5 weeks for the transplanted tumors to grow up to 100 mm3, when treatment with MO-I-1182 (10 mg/kg, orally, every other day) was initiated. The mice were followed up for 5 weeks until the tumors grew up to 1000 mm3.
(F) Antitumor effects of the SMI on pulmonary metastasis administered with orally formulated (capsules) MO-I-1182 blocks pulmonary micro-/macro-metastases (arrows) in mice of F5 generation PDX derived from Patient B.
(G-I) Expression profiling of ASPH, Active Notch1 and MMPs detected by IHC and qRT-PCR in representative mice of F5 generation PDX model treated with SMI vs. DMSO control.
