Abstract
Purpose:
Pathological angiogenesis and apoptosis evasions are common hallmarks of cancer. A different approach to the antitumor effect of parasitic diseases caused by certain protozoans and helminthes had been adopted in recent years as they can affect many cancer characteristics. The present work is an attempt to assess the effect of gamma radiation-attenuated Toxoplasma gondii ME49 as an antiapoptotic and angiogenic regulator modifier on tumor growth aimed at improving cancer protective protocols.
Methods:
Attenuated Toxoplasma gondii ME49 was administered orally to mice 2 weeks before inoculation with Ehrlich ascites carcinoma to allow stimulation of the immune response. Hepatic histopathology and immune responses were determined for each group.
Results:
Marked suppression of the tumor proliferation with induction of long-lasting immunity by stimulating interferon γ and downregulating transforming growth factor β. The level of tumor promoting inflammatory markers (STAT-3 and tumor necrosis factor α), the angiogenic factors (vascular endothelial growth factor A, integrin, and matrix metallopeptidase 2 and matrix metallopeptidase 9), as well as nitric oxide concentration were significantly decreased. This was collimated with an improvement in apoptotic regulators (cytochrome-c, Bax, Bak, and caspase 3) in liver tissues of vaccinated mice group compared to Ehrlich ascites carcinoma-bearing one. Moreover, the histopathological investigations confirmed this improvement.
Conclusion:
Hence, there is an evidence of potency of radiation attenuated Toxoplasma vaccine in immune activation and targeting tumor cell that can be used as a prophylactic or an adjuvant in combination with chemotherapeutic drugs.
Keywords: Toxoplasma gondii, gamma radiation, IFN-γ, STAT-3, VEGF-A, angiogenic, apoptotic regulator
Introduction
Cancer is an irregular cell growth arising from oncogene activation caused by inactivation of the tumor suppressor gene or accumulation of inherited and/or acquired genetic mutations. This contributes to dysregulation of the usual cellular system of division, differentiation, and proliferation-death balance which finally lead to population of cells that can metastasize and invade tissues in remote sites causing significant morbidity.1
In conjunction with current therapies, there is still a strong need for the development of prophylactic and therapeutic agents to enhance the immune response and further combat cancer. In particular, a novel protection that provide long-term defense against highly aggressive metastatic cancer and recurrence of tumors will be crucial for improving patient’s survival. A different approach to the antitumor effect of parasitic diseases had been adopted in recent years however the mechanisms involved remain unknown.2
The beneficial effects of certain parasites on tumorigenesis range from apoptosis induction, activation of the immune response, avoidance of metastases, and angiogenesis. In addition, inhibition of proliferative signals and control of inflammatory reactions that promotes cancer.3
Toxoplasma gondii tumor-bearing mice therapy facilitated long-lasting tumor-free survival in highly aggressive murine tumor models as ovarian cancer, melanoma, and pancreatic cancer. This remarkably effective primary tumor defense is activated and orchestrated by modulation of tumor-associated myeloid cells by T gondii, which results in the reduction of tumor-associated immune suppression, thus facilitating the activation of substantial antitumor immune responses.2
An effective immunotherapy recently reported for known tumors is that induced by the attenuated nonreplicating T gondii.4 Previous studies demonstrated immunotherapeutic potency of treatment with nonreplicating attenuated T gondii uracil auxotroph (CPS) that can reverse tumor-associated immunosuppression.5 Also, it targets tumor-associated myeloid cells and stimulates more effective immunity to primary pancreatic cancer so generates long-lasting immunity to recurrences.6
Antitumor effects of T gondii antigens showed significant growth inhibition against malignant glioma7 and WEHI 164 fibrosarcoma8 in mice model. Mohamadi et al demonstrated that anti-T gondii antibodies can attach mouse cancer cell lines lymphocytes.9
Another study using avirulent T gondii ME49 strain revealed its therapeutic efficacy to inhibit tumor growth in the Lewis lung carcinoma mouse model through antiangiogenic activity and the induction of Th1 immune responses.10 Also, it has been shown that it is able to generate antitumor therapeutic immunity against ovarian cancer.11
Hiramoto et al reported that attenuating T gondii tachyzoites using gamma radiation induced parasite sterilization without cell death, retaining an intact structure, physiology, and unchanged proteins.12 Recent model using gamma radiation-attenuated T gondii ME49 vaccine has been demonstrated immune protection against ovarian infiltration in mice-bearing Ehrlich ascites carcinoma (EAC).13
Hence, this study aimed to evaluate the antitumor modality of gamma radiation-attenuated T gondii as a proapoptotic and antiangiogenic regulator in liver tissues of EAC-bearing mice.
Materials and Methods
Chemicals
In this study, chemicals and reagent were obtained from Sigma-Aldrich Chemical Co.
Irradiation of parasites
Avirulent T gondii strain ME49 cysts obtained from the National Center of Research were irradiated with attenuating dose of 0.4 kGy gamma radiation at the National Center for radiation Research and Technology, Cairo, Egypt. This was done using Indian Cobalt-60 gamma chamber 4000 A irradiator at a dose rate of 2.5 Krad/h at the time of experimentation.12
Tumor Transplantation
Ehrlich ascites carcinoma cell line was used by intraperitoneal (ip) inoculation in albino mice as a model of carcinoma. The parent line was given as a gift from the University of Cairo’s Egyptian National Cancer Institute. Human breast cancer is the source of EAC cells in female Swiss albino mice when adjusted to develop. Through ip injection of 2.5 million cells per mouse (counted by the bright line hemocytometer), the cell line was preserved. The physiological sterile saline solution was used for injection and dilution. In order to develop EAC, 0.2 mL EAC cells (2.5 × 106 cells /mouse) were inoculated intraperitoneal in female mouse.
Vaccination of Animals
Gamma radiation-attenuated avirulent ME49 T gondii cysts (0.4 KGy) were given orally to mice (10 cysts each) 2 weeks before tumor cell inoculation.10
Animals Categorize
In this study, we used 40 female Swiss albino mice weighing about 25 g. All the experiments were conducted under national research center guidelines for the use and care for laboratory animals and were approved by an independent ethics committee of the NCRRT. They were categorized into 4 equal groups of 10 mice each as follows:
Group 1: Control (C): Mice neither treated nor irradiated.
Group 2: (T gondii ME49): Mice orally injected with gamma radiation-attenuated T gondii.
Group 3: (EAC): Mice bearing EAC.
Group 4: (EAC + T gondii ME49): Mice were first orally subjected to radiation-attenuated T gondii then were intraperitoneally injected 2 weeks later with EAC.
Mice were anesthetized with diethyl ether at the end of experiment. The liver tissues were collected for biochemical and histopathological investigations.
Quantitative Real-Time Polymerase Chain Reaction
RNA isolation and reverse transcription
RNA was extracted from the homogeneous liver tissue using the RNeasy Plus Mini Kit (Qiagen, Venlo) as directed by the manufacturer. ADNase-on-column treatment given with the package removed genomic DNA. The RNA concentration was measured using the Nano Drop ND-1000 spectrophotometer (Thermo Fisher scientific) spectrophotometrically at 260 nm and the purity of RNA was tested using the absorbance ratio at 260/280 nm. Electrophoresis tested the integrity of RNA on 2% agarose gels. Using the Reverse Transcription Kit (Promega, Leiden), RNA (1 μg) was used in the subsequent complementary DNA (cDNA) synthesis reaction. To avoid secondary structures, the total RNA was incubated at 70 °C for 10 minutes. MgCl2 (25 mM), reverse transcriptase buffer (10×), deoxynucleoside triphosphate (10 mM), oligod (t) primers, RNase inhibitor (20 U), and AMV reverse transcriptase (20 U/μL) were applied to the RNA. This mixture was incubated for 1 hour at 42 °C.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction was performed on an optical 96-well plate with a quick sequence detection system ABI PRISM 7500 (Applied Biosystems) and uniform cycling conditions of 40 cycles of 15 seconds at 95 °C and 60 seconds at 60 °C after an initial denaturation stage at 95 °C for 10 minutes. Each reaction of 10 µL produced 5 µL SYBR Green Master Mix (Applied Biosystems), 0.3 µL gene-specific forward, reversing primers (10 μM), 2.5 µL cDNA, and 1.9 µL nuclease-free water. Table 1 displays the sequences of the polymerase chain reaction primers used for each gene. The data were analyzed using the ABI Prism sequence detection system software and quantified using the PE Biosystems (Foster City) v1.7 sequence detection software. The comparative threshold process approach was used to measure the relative expression of studied genes. All values have been standardized under GAPDH endogenous regulation.14
Table 1.
Primers Used for qRT-PCR.
| Primer | Sequence |
|---|---|
| STAT-3 | Forward primer: 5′-ACCCAACAGCCGCCGTAG-3′ Reverse primer: 5′-CAGACTGGTTGTTTCCATTCAGAT-3′ |
| VEGFA | Forward primer: 5′-ATCTGCATGGTGATGTTGGA-3′ Reverse primer: 5′-GGGCAGAATCATCACGAAGT-3′ |
| GAPDH | Forward: 5′-CTCCCATTCTTCCACCTTTG-3′ Reverse: 5′-CTTGCTCTCAGTATCCTTGC-3′ |
Abbreviations: VEGFA, vascular endothelial growth factor A; qRT-PCR, quantitative real-time polymerase chain reaction.
ELISA Detection
Enzyme-linked immune sorbent assay (ELISA) for levels of interferon γ (INF-γ), transforming growth factor β (TGF-β), tumor necrosis factor α (TNF-α), and caspase 3 were determined by using ELISA Kit (INF-γ, R & D systems), (MyBioSource Kit for TGF-β and TNF-α), and RayBiotech ELISA Kit for caspase 3. Their levels were evaluated according to the manufacturer instructions on the supernatants of sample tissue homogenates.
Nitric Oxide Determination
Nitric oxide (NO) level in the liver tissues was determined colorimetric as nitrite by Griess reaction.15
Western blot analysis of integrin, MMP2, MMP9, cytochrome-c, Bax and, Bak proteins in hepatic tissue homogenate
Liver tissue protein was extracted using Trizol reagent and protein concentration was quantified according to the study by Bradfor.16 Twenty µg of protein per lane were isolated using 10% acrylamide gels by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to the membranes of polyvinylidene difluoride. Membranes were incubated with blocking solution at room temperature for 2 hours (5% nonfat dried milk in 10 mM Tris-HCl, pH 7.5; 100 mM NaCl; and 0.1% Tween 20), then incubated at 40 °C overnight with primary integrin antibody, matrix metallopeptidase (MMP) 2, MMP9, cytochrome-c, Bax, and Bak proteins with β-actin as regulation. After washing 3 times in 10 mM Tris-HCl, pH 7.5; 100 mM NaCl; and 0.1% tween 20, membranes were incubated at room temperature for 2 hours with the secondary monoclonal antibody combined with horseradish peroxidase, and then membranes were washed 4 times with the same washing buffer. Membrane was developed and visualized by chemiluminescence using the detection kit of Invitrogen (catalog # AHO1202) according to the protocols of the manufacturer, and then exposed to X-ray film. Protein quantification was achieved using laser densitometer scanning (BiomedInstrumentInc, Fullerton).
Histopathological Study
Autopsy samples were taken from mice’s liver in various groups and set for 24 hours in 10% formol saline. Washing in tap water was performed and serial alcohol dilutions (methyl, ethyl, and pure ethyl) were used for dehydration. Specimens were washed in xylene and stored for 24 hours in a 56-degree paraffin in a hot air oven. Paraffin bees wax blocks of tissue were prepared by slidge microtome for sectioning at 4 microns thickness. For routine inspection through the light electric microscope, the tissue parts obtained were collected on glass slides, deparaffinized, and stained with hematoxylin and eosin dye.17
Statistics
Statistical analysis was carried out using the Social Science Statistical Package version 20.0 for windows.
Results
Effects of Gamma Radiation-Attenuated T gondii on Mice Immune Regulators
Table 2 represents the impact of vaccination with attenuated T gondii on mice immune response (IFN-γ and TGF-β concentration). The results obtained revealed a significant increase in IFN-γ followed by a significant downregulation of TGF-β concentration in T gondii-vaccinated mice compared with their equivalents in EAC-bearing mice.
Table 2.
Effects of Gamma Radiation-Attenuated Toxoplasma gondii on Hepatic Levels of IFN-γ and TGF-β Levels in Different Animal Groups.a
| C | Toxoplasma gondii | EAC | EAC + Toxoplasma gondii | |
|---|---|---|---|---|
| IFN-γ, ng/mL | 80.88 ± 0.73b | 71.38 ± 1.22b | 118.48 ± 2.26a | 200.18 ± 4.44a,b |
| TGF-β, ng/mL | 115.26 ± 1.58b | 111.96 ± 0.75b | 239.62 ± 5.07a | 160.64 ± 5.19a,b |
Abbreviations: C, normal control mice; EAC, mice inoculated with Ehrlich ascites carcinoma; IFN-γ, interferon γ; SE, standard error; TGF-β, transforming growth factor β, T gondii, mice treated with gamma attenuated Toxoplasma gondii.
Each value represents the mean of 6 records ± SE.
a Significant difference versus control group at P ≤ .05.
b Significant difference versus EAC group at P ≤ .05.
Effect of Gamma Attenuated T gondii Administration on Tumor Promoting Inflammatory Markers
Compared to normal control mice, substantial upregulation was shown in the level of TNF-α and the expression of STAT3 gene, respectively, in EAC-bearing mice. On the other hand, a significant decline in their levels was detected in vaccinated group (Table 3).
Table 3.
Effect of Gamma Attenuated Toxoplasma gondii on Hepatic Levels of TNF-α Level and STAT-3 Expression in Different Animal Groups.a
| C | Toxoplasma gondii | EAC | EAC + Toxoplasma gondii | |
|---|---|---|---|---|
| TNF-α, ng/mL | 47.98 ± 1.38b | 40.9 2 ± 0.95b | 142.8 ± 2.11a | 69.26 ± 5.4a,b |
| STAT-3 | 1.01 ± 0.01b | 1.7 ± 0.11b | 9.11 ± 0.66a | 4.7 ± 0.26a,b |
Abbreviations: C, normal control mice; EAC, mice inoculated with Ehrlich ascites carcinoma; SE, standard error; T gondii, mice treated with gamma attenuated Toxoplasma gondii; TNF-α, tumor necrosis factor α.
Each value represents the mean ± SE (n = 6).
asignificantly different from control.
bsignificantly different from EAC.
Impact of T gondii Administration to Mice on Angiogenic Parameters
It has been shown that mice with EAC tumors showed significant increases in the gene expression of vascular endothelial growth factor A (VEGF-A) and protein expression of integrin, MMP-2 and MMP-9, and NO concentration, respectively, relative to normal control. While vaccination with gamma radiation-attenuated T gondii resulted in a significant reduction in their expressions in liver tissue (Table 4).
Table 4.
Impact of T gondii on Angiogenic Regulators in Different Mice Groups.a
| C | T gondii | EAC | EAC + T gondii | |
|---|---|---|---|---|
| VEGF | 1.00 ± .015b | 1.36 ± 0.16b | 10.19 ± 0.2a | 5.34 ± 0.28a,b |
| Integrin | 1.02 ± 0.01b | 1.15 ± 0.05b | 7.06 ± 0.29a | 3.66 ± 0.3a,b |
| MMP2 | 1.014 ± 0.015b | 1.12 ± 0.05b | 5.53 ± 0.31a | 3.02 ± 0.22a,b |
| MMP9 | 1.00 ± 0.015b | 1.17 ± 0.04b | 6.06 ± 0.29a | 2.94 ± 0.47a,b |
| NO, nmol/mL | 10.16 ± 0.6b | 8.86 ± 0.3b | 32.32 ± 2.94a | 14.83 ± 0.35a,b |
Abbreviation: C, normal control mice; EAC, mice inoculated with Ehrlich ascites carcinoma; MMP, matrix metallopeptidase; NO, nitric oxide; SE, standard error; T gondii, mice treated with gamma attenuated Toxoplasma gondii; VEGF, vascular endothelial growth factor.
Each value represents the mean ± SE (n = 6).
asignificantly different from control.
bsignificantly different from EAC.
Impact of T gondii in Apoptosis Stimulation in Different Mice Group
Table 5 shows a significant decrease in the expression of proapoptotic molecules Bax, Bak, and cytochrome-c with major downregulation in caspase 3 activity in EAC-bearing mice compared to normal control. On the other hand, a significant pronounced elevation in the expression of Bax, Bak, and cytochrome-c accompanied by a significant increase in caspase 3 activity in vaccinated group (EAC + T gondii) relative to EAC-bearing mice was detected.
Table 5.
Effect of T gondii in Apoptotic Regulator Molecules in Different Mice Group.a
| C | T gondii | EAC | EAC + T gondii | |
|---|---|---|---|---|
| Bax | 1.00 ± 0.09b | 1.45 ± 0.13b | 0.24 ± 0.02a | 1.73 ± 0.08a,b |
| Bak | 1.014 ± 0.015b | 1.28 ± 0.07b | 2.24 ± 0.2a | 4.66 ± 0.13a,b |
| Cytochrome-c | 1.00 ± 0.13b | 1.42 ± 0.03b | 0.44 ± 0.03a | 4.35 ± 0.22a,b |
| Caspase 3, pg/mL | 2.08 ± 0.27b | 1.77 ± 0.09b | 0.56 ± 0.01a | 3.45 ± 0.21a,b |
Abbreviations: C, normal control mice; EAC, mice inoculated with Ehrlich ascites carcinoma; SE, standard error; T gondii, mice treated with gamma attenuated Toxoplasma gondii.
Each value represents the mean ± SE (n = 6).
a significantly different from control.
bsignificantly different from EAC.
Impact of Attenuated T gondii Vaccine on Hepatic Histopathological Changes of EAC-Bearing Mice
Photomicrographic examinations of control mice liver tissue demonstrate normal histological structure (Figure 1A and B). Liver showed average central veins surrounded by hepatocytes arranged in single-cell cords with intervening blood sinusoids. Average portal tracts with average bile ducts and average portal veins were observed in attenuated T gondii mice group (Figure 2). However, photomicrograph of EAC-bearing mice displayed compactness of the tumor cells scattered within the liver tissues and aggregated in focal manner infiltrating and penetrating the tissue, liver showed surrounding viable tumor tissue with intrahepatic cellular infiltrate, dilated congested central veins with marked perivenular inflammatory infiltrate, hepatocytes with marked hydropic degeneration, apoptosis and binucleation, and areas of hemorrhage (Figure 3). On the other hand, the histopathological investigation performed on liver tissue sections of EAC + T gondii mice showed a great destruction of tumor tissue represented by the appearance of dead and necrotic cells, liver showed surrounding tumor tissue with marked areas of necrosis showing karyorrhectic fragments, apoptotic tumor cells and inflammatory infiltrate, and average central veins with surrounding markedly apoptotic hepatocytes with areas of necrosis and inflammatory infiltrate (Figure 4).
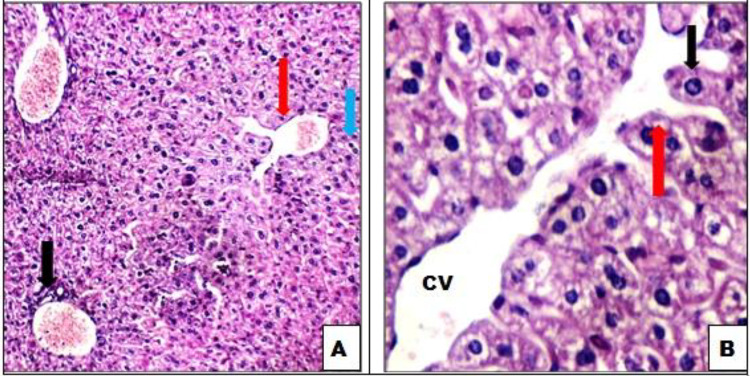
Figure 1.
A, control mice liver tissue showing average portal tract (black arrow), average central vein (red arrow), and average hepatocytes (blue arrow; hemotoxylin and eosin ×200). B, Average central vein (CV) and average hepatocytes arranged in single-cell cords (black arrow) with average intervening blood sinusoids (red arrow; hemotoxylin and eosin ×400).
Figure 2.
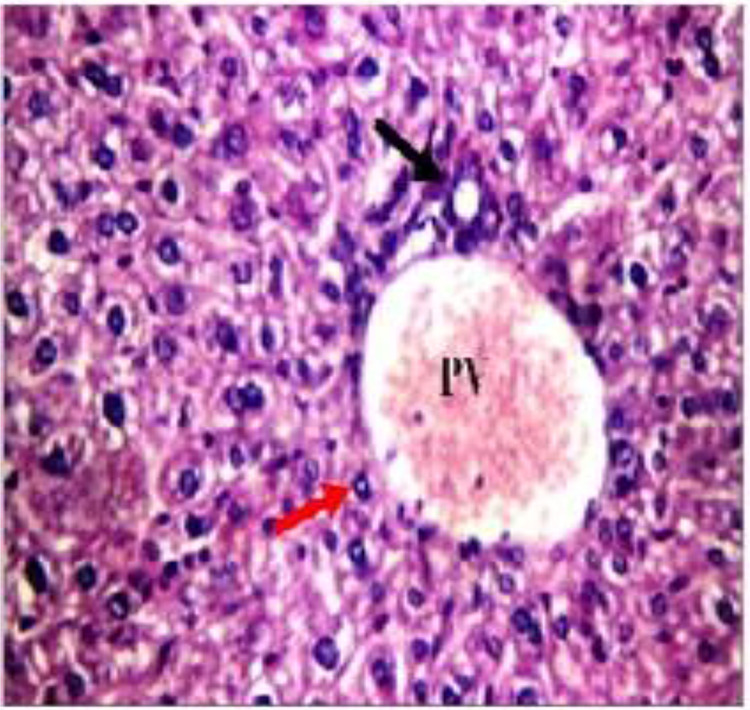
Liver tissue of gamma attenuated Toxoplasma gondii group showing average portal tract with average portal vein (PV), average bile duct (black arrow), and average hepatocytes in periportal area (red arrow; hemotoxylin and eosin ×400).
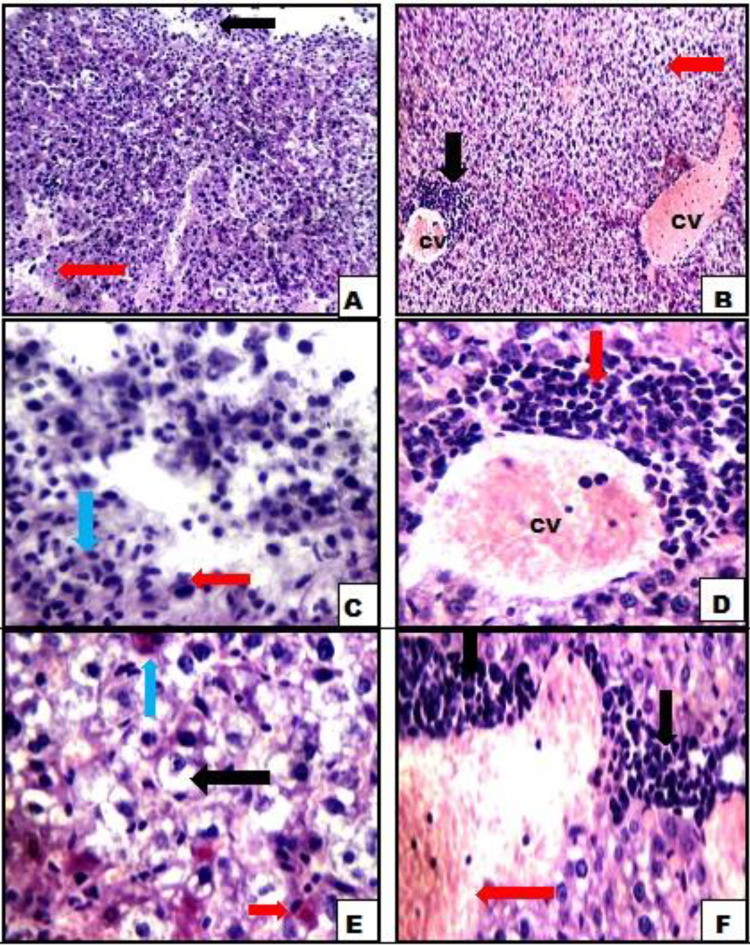
Figure 3.
A, Liver tissue of Ehrlich ascites carcinoma (EAC)-bearing mice with surrounding viable tumor tissue (black arrow) and intrahepatic cellular infiltrate (red arrow). B, Dilated congested central veins (CV), perivenular inflammatory infiltrate (black arrow), and hydropic degeneration of hepatocytes (red arrow). C, High power view showing liver tissue (blue arrow) with surrounding viable tumor tissue composed of markedly pleomorphic cells with hyperchromatic nuclei (red arrows). D, Another view showing dilated congested CV with marked perivenular inflammatory infiltrate (red arrow). E, Another view showing hepatocytes with marked hydropic degeneration (black arrows), apoptosis (red arrows), and binucleation (blue arrow). F, Another view showing marked inflammatory infiltrate (black arrows) with areas of hemorrhage (red arrows; A and B, hemotoxylin and eosin ×200; C-F hemotoxylin and eosin ×400).
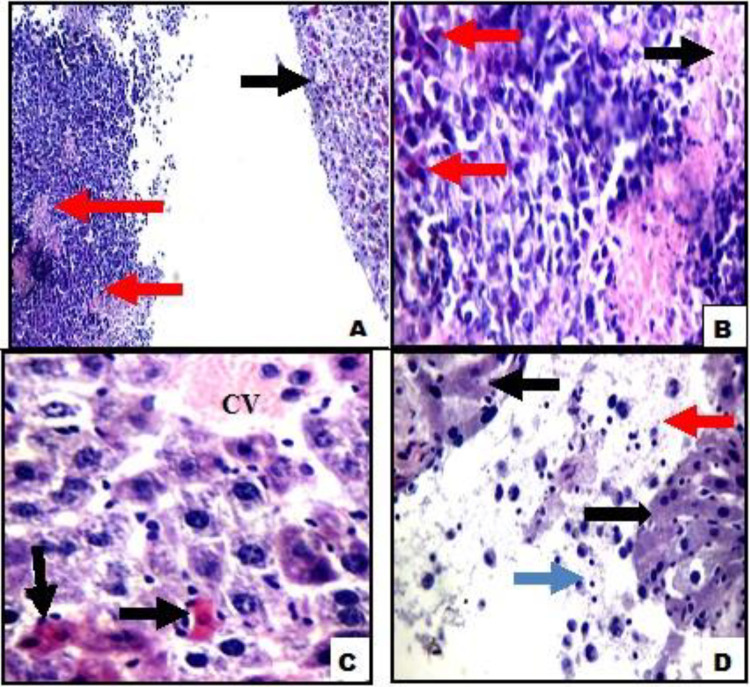
Figure 4.
A, Liver tissue of Ehrlich ascites carcinoma (EAC) + T gondii showing markedly apoptotic cells (black arrows) with surrounding tumor tissue showing areas of necrosis (red arrows; hemotoxylin and eosin ×200). B, High power view showing tumor tissue with areas of necrosis showing karyorrhectic fragments (black arrows) and apoptotic tumor cells (red arrows). C, Another view showing average central vein (CV) with surrounding markedly apoptotic hepatocytes (black arrows). D, Another view showing liver tissue (black arrows), with surrounding markedly necrotic tumor tissue (red arrows) with inflammatory infiltrate (blue arrows; B-D hemotoxylin and eosin ×400).
Discussion
Due to the great importance of the immune system in cancer development, many researches had been made to develop tumor-driven immune therapies with the goal of increasing the antitumor immune response and thereby eradicating the neoplastic in advance. The use of helminthes and protozoa to reactivate immune responses was documented among antitumor therapies, such is the case of T gondii.3 In an attempt to improve cancer protective protocols, this study was undertaken to evaluate the antitumor effect of gamma radiation-attenuated T gondii ME49 against EAC in female mice. In order to explore the underlying mechanisms by which the attenuated Toxoplasma suppressed the growth of tumor, different molecular target proteins were analyzed.
Our result showed a significant decrease in the concentration of IFN-γ followed by significant increase in TGF-β concentration in EAC-bearing mice compared to normal. Lactate acidosis, a hallmark of malignant tissue, adversely controls the development of IFN-γ by natural killer (NK) cells in the context of tumor transformation resulting in a gradual loss of expression in NK cells18 followed by a significant increase in TGF-β.19 The rise in TGF-β concentration could be interpreted in terms of its role in tumorgenesis, and TGF-β1 was shown to increase angiogenesis, an important component of tumor progression.20
Vaccination of EAC-bearing mice with gamma radiation-attenuated T gondii produced a significant upregulation of IFN-γ and downregulation of TGF-β enhancing the antitumor immune response. Toxoplasma gondii triggers a heavy polarization of Th1 responses in its host, with an increase in the production of IFN-γ.21,22 It induces NK cells and T cells to produce IFN-γ, thereby increasing cytotoxic cell activity and encouraging an antitumor immune response.23 The excretory-secretory antigens induces apoptosis in T regulatory lymphocytes, which are essential to produce TGF-β cells.24 Therefore, it can be assumed that these antigens inhibit TGF-β elevation by apoptosis induction in T regulatory lymphocytes. Inhibition of TGF-β maintains the role of highly activated, in vitro expanded NK cells in the tumor environment.19
The data in the present study revealed that STAT-3 and TNF-α (the angiogenic) were upregulated in the time of decreases in the apoptotic mediators (caspase 3, cytochrome c, Bax, and Bak). This may interpreted as a result of increasing IFN-γ concentration in attenuated T gondii-vaccinated group.
STAT5 has been shown to drive the development of IFN-γ which in turn reduces the activation of STAT-3.25,26 The increases in TNF-α which is a central cytokine in the development of tumors in the skin of the mouse and, quite likely, in human carcinogenesis, could be contributed to activation of STAT-3.27
Activation of key inducers of proinflammatory cascades, including the transcription factors STAT-3 and nuclear factor κβ, leads to an increase in cell proliferation, apoptotic evasion, invasion and metastasis, as well as angiogenesis, all of which are well-established cancers.28 Tumor necrosis factor α is a key player in cancer-related inflammation and promotes angiogenesis and metastasis in clinically models of human and mouse epithelial carcinoma tumor through various mechanisms, including tumor inflammation, tumor angiogenesis, and epithelial-mesenchymal transition (EMT).29 In addition, it is a major inflammatory mediator that causes multiple changes in the gene expression of endothelial cells, including the activation of adhesion molecules, integrins, and MMPs, consequently, serves as an autocrine growth factor for angiogenesis of tumors.30
Moreover, the obtained data in Table 4 and Figure 5 displayed a significant elevation in the expression of the angiogenic markers VEGF-A, MMP-2, and MMP-9. It was reported that IFN-γ suppresses VEGF production in vitro and in vivo and thus inhibits angiogenesis.31 STAT-3 directly regulates the transcription of the VEGF gene (the most potent proangiogenic stimulus that plays a critical role in tumorgenesis by inducing angiogenesis and metastasis of tumors) and induces its overexpression in various tumors.32 The VEGF promoter contains various binding sites for the transcription factor, including STAT-3 sites and the hypoxia-inducible factor 1(HIF-1). STAT-3’s physical contact with HIF-1 guides VEGF transcription activation by binding with the VEGF promoter.33 Increased expression of MMPs notable in our results is predictive of tumor aggressiveness and metastasis.
Figure 5.
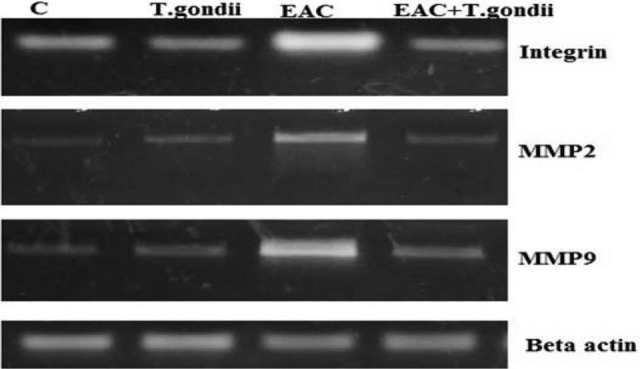
Western immune blotting analysis of angiogenic factors integrin, matrix metallopeptidase (MMP) 2, and MMP9 protein expressions in different mice groups. MMP2, matrix metalloproteinase 2; MMP9, matrix metalloproteinase 9; Betaactin, “housekeeping” protein, used as a loading control; C, normal control mice; EAC, mice inoculated with Ehrlich ascites carcinoma; T gondii, mice treated with gamma attenuated Toxoplasma gondii.
Recently, MMPs (a family of enzymes which are key players in the processes of matrix remodeling) had have been considered to be an important factor in triggering EMT. They allow tumor cells to alter the extracellular matrix and release cytokines, growth factors, and other cell surface molecules, thereby facilitating the progression of protease-dependent tumors.34 It has been documented that the expression of STAT-3 improves invasiveness in less invasive melanoma cells by increasing expression and activity of MMP-2.35 Tumor necrosis factor α has already been shown to be involved in cell–cell contact and in breast cancer cell coculture and tumor-associated macrophages; macrophage-derived TNF-α has increased MMP-2 and MMP-9 rates in tumor cells.36 Cancer cells due to metastasis to distant organs have been shown to express elevated MMP-9 levels. Matrix metallopeptidase 9 is therefore not only critical in recognizing signs of invasion and diagnosis but also a promising therapeutic goal to prevent invasion and metastasis of cancer.37
Further, the significant increase in NO concentration of EAC-bearing mice might be due to TNF-α overexpression which has been shown to be an NO synthesis mediator.38 The earliest stages of angiogenesis are characterized by NO-induced vasodilatation and increased vascular permeability of pre-existing capillaries or postcapillary venules in response to VEGF,39 which overexpressed by IFN-γ, STAT-3, and TNF α.
The result of this study revealed that vaccination of EAC-bearing mice with radiation-attenuated T gondii resulted in decrease in angiogenesis (downregulation of TNF-α, STAT-3, VEGF, integrin expression, and NO concentration; Tables 3 and 4), invasion (reduction of serum MMP-2 and -9 activities; Figure 5), and enhancing apoptosis (induction of caspase 3, cytochrome C, Bax, and Bak; Figure 6). This result is in line with Khan et al who found that inactivation of STAT-3 allows cancer-related inflammation to be suppressed and the immune suppressive tumor microenvironment to be decreased, resulting in activation of antitumor immunity and cytototoxic T-cell effector functions.40 There is proof that T gondii prevents and induces degradation of STAT phosphorylation. Certain data suggest that STAT nuclear translocation is prevented by the parasite.41 Zimmermann et al provided evidence that T gondii inhibits STAT signals in RAW264.7 cells by inducing the negative suppressor of cytokine signaling 1 regulator.42 Even, during TLR4 stimulation, it was involved in down modulation of TNF-α production.43 Tumor-related neovascularization is produced by the angiogenesis process, which is necessary to supply the tumor cells with nutrients and oxygen for their neoplastic development. Protozoan infections have been documented to be important in this process, especially during the acute phase of infection with T gondii, the development of type II IFNs and cytokines with antiangiogenic properties is growing. In a model of melanoma in vivo and a model of Lewis lung cancer, treatment with attenuated avirulent T gondii prevented neoplastic development by blocking neovascularization by hypoxia induction and vascular necrosis.3,10,44 Nevertheless, in addition to lysing neoplastic cells, immune cells often secrete soluble factors that may indirectly inhibit tumor growth, for example through angiogenesis suppression. It is supported by the fact that plasma from infected but not control mice prevents the formation of tube-like structure by endothelial cells in vitro on Matrigel surface. In addition, several cytokines developed systemically during acute toxoplasmosis had antiangiogenic properties, most notably type I and type II IFNs.45 It is also known that IFN-γ stimulates IFN-γ-inducible protein 10, a chemokine involved in both T gondii resistance46 and angiogenesis suppression.47
Figure 6.
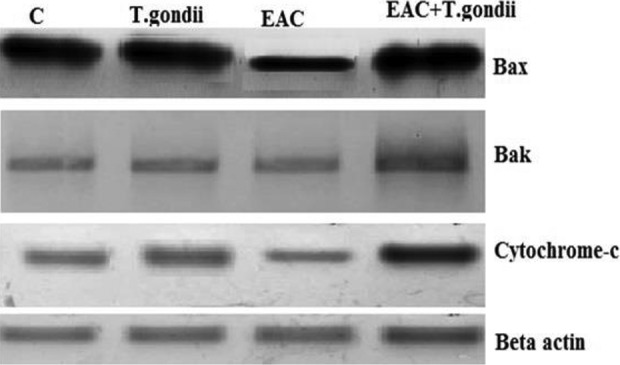
Western immunoblotting analysis of apoptotic factors Bax, Bak, and cytochrome-c protein expressions in different mice groups. Bax: Bcl-2-associated X protein; Bak: Bcl-2 homologous antagonist killer. Betaactin: “housekeeping” protein, used as a loading control. C: normal control mice. EAC: mice inoculated with Ehrlich ascites carcinoma. T gondii: mice treated with gamma attenuated Toxoplasma gondii.
The angiogenic and apoptotic regulator disruption leads to tumor proliferation and development. Neovascularization increases the tumor’s ability to grow and its invasiveness and metastatic potential. Our results demonstrated a significant decline in the level of apoptotic molecule (Bax, Bak, cytochrome-c, and caspase 3) in EAC-bearing mice compared to normal control (Table 5 and Figure 6). In order to eliminate cancer cells, most antineoplastic chemotherapies rely on activation of the intrinsic apoptotic pathway mediated by mitochondria. The main effector proteins for intrinsic apoptosis, BAX, BAK, and/or BOK, form (once activated) pores in the outer membrane of the mitochondria (MOMP) and induce permeabilization of the outer MOMP. The subsequent release of cytochrome c enables the “apoptosome” complex to be assembled. It facilitates activation of caspase 9, the intrinsic apoptotic pathway’s prototypical initiator caspase. In addition, caspase 9 stimulates the effector caspases 3 and 7, both apoptosis performers.48,49 In many cancers, however, proteins that control the permeability of the outer mitochondrial membrane to release cytochrome c are defective via mutations.50
Ehrlich ascites carcinoma-vaccinated group revealed high significant increase in the level of apoptotic molecule (Bax, Bak, cytochrome-c, and caspase 3) compared to EAC-bearing mice (Figure 6). Caspase 3-mediated apoptosis is a major focus in the field of cancer growth inhibition, as proteolytic caspase cascade activation is a critical component of apoptotic cell death.51 Therefore, current cancer treatments, including chemotherapy and immunotherapy, also work primarily by encouraging tumor cell apoptosis.52 Protozoa and helminthes were identified as apoptosis inducers, a mechanism of survival, in immune system cells and epithelial cells.3 Toxoplasma gondii tachyzoite has certain antitumor active substances that can be secreted after infecting the host cell and contributing to apoptosis of the cell.53 This was in agreement with Wang and Gao who found that tachyzoites of T gondii had an inhibiting effect on the proliferation of human HCC H7402 cells and increased the inhibition rate of cell growth as they increased.54 Toxoplasma gondii tachyzoite can lead to host cell apoptosis and block the cell cycle to phase G2/M by regulating cyclineB1 expression. The quantity of expression of caspase 3 protein increased as the concentration of T gondii tachyzoite increased. Various studies in mouse and human cells have shown that T gondii modulates cancer cells’ apoptotic response by upregulating cancer cell apoptosis.4
Finally, it can be concluded that gamma radiation-attenuated Toxoplasma ME49 vaccine induces marked suppression of tumor proliferation with induction of long-lasting immunity by stimulating IFN-γ and TGF-β downregulation, with significant decrease of promoting inflammatory markers (STAT-3 and TNF-α), angiogenic factors (VEGF-A, integrin, and MMP-2 and MMP9), and NO concentration. This was collimated with an improvement in apoptotic regulators (cytochrome-c, Bax, Bak, and caspase 3) and amelioration of histopathological changes in liver tissues. Further studies are required to explore the mechanistic aspect of antiapoptotic and antiangiogenic effect that may be useful for directing the application of a selective vaccine delivery in cancer protection.
Acknowledgments
Thanks to all the authors.
Abbreviations
- cDNA
complementary DNA
- EAC
Ehrlich ascites carcinoma
- ELISA
enzyme-linked immune sorbent assay
- EMT
epithelial–mesenchymal transition
- HIF-1
hypoxia-inducible factor 1
- IFN-γ
interferon γ
- Ip
intraperitoneal
- MMP
matrix metallopeptidase
- MOMP
membrane of the mitochondria
- NK
natural killer
- NO
nitric oxide
- TGF-β
transforming growth factor β
- TNF-α
tumor necrosis factor α
- VEGF-A
vascular endothelial growth factor A
Authors’ Note: The animal procedure was approved by the Ethics Committee of the National Center for Radiation Research and Technology.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nermeen M. Elbakary  https://orcid.org/0000-0002-0128-3159
https://orcid.org/0000-0002-0128-3159
References
- 1. Upadhyay RK. Use of animal venom peptides/toxins in cancer therapeutics. Curr Trends Biomed Eng Biosci. 2018;16(1):555945. [Google Scholar]
- 2. Costa A, Zorgi NE, Nascimento N, et al. Gamma irradiation of Toxoplasma gondii protein extract improve immune response protection in mice models. Biomed Pharmacother. 2018;106(10):599–604. [DOI] [PubMed] [Google Scholar]
- 3. Callejas BE, Martínez-Saucedo D, Terrazas LI. Parasites as negative regulators of cancer. Bio Sci Rep. 2018;38(5):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mammari N, Halabi MA, Yaacoub S, et al. Toxoplasma gondii Modulates the Host Cell Responses: An Overview of Apoptosis Pathways. BioMed Res Int. 2019;2019(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox BA, Sanders KL, Bzik DJ. Non replicating Toxoplasma gondii reverses tumor associated immunosuppression. Oncoimmunology. 2013;2(6):e26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanders KL, Fox BA, Bzik DJ. Attenuated Toxoplasma gondii therapy of disseminated pancreatic cancer generates long-lasting immunity to pancreatic cancer. Oncoimmunology. 2016;5(4):e1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choo JD, Lee JS, Kang JS, Lee HS, Yeom JY, Lee YH. Inhibitory effects of Toxoplasma antigen on proliferation and invasion of human glioma cells. J Korean Neurosurg Soc. 2005;37(4):129–136. [Google Scholar]
- 8. Darani HY, Shirzad H, Mansoori F, Zabardast N, Mahmoodzadeh M. Effects of Toxoplasma gondii and Toxocaracanis antigens on WEHI 164 fibrosarcoma growth in a mouse model. Korean J Parasitol. 2009;47(7):175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohamadi F, Shakibapour M, Sharafi SM, Andlib AR, Toloue S, Darani HY. Anti-Toxoplasma gondii antibodies attach to mouse cancer cell lines but not normal mouse lymphocytes. Biomed Rep. 2001;9(10):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JO, Jung SS, Kim SY, et al. Inhibition of Lewis lung carcinoma growth by Toxoplasma gondiithroughinduction of Th1 immune responses and inhibition of angiogenesis. J Korean Med Sci. 2007;22(3):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baird JR, Fox BA, Sanders KL, et al. Avirulent Toxoplasma gondii generates therapeutic antitumor immunity by reversing immunosuppression in the ovarian cancer microenvironment. Cancer Res. 2013;73(5):3842–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiramoto RM, Galisteo A, Jr, Do Nascimento N, et al. 200 Gy sterilized Toxoplasma gondiitachyzoites maintain metabolic functions and mammalian cell invasion, eliciting cellular immunity and cytokine response similar to natural infection in mice. Vaccine. 2002;20(8):2072–2081. [DOI] [PubMed] [Google Scholar]
- 13. Hafez EN, Hanan MG, Youssef HMG, El Kabany HA. Vaccination with gamma radiation-attenuated Toxoplasma gondii protects against ovarian infiltration in mice-bearing Ehrlich ascites carcinoma. Int J Radiat Biol. 2020;1–9. doi:10.1080/09553002.2020.1739772 [DOI] [PubMed] [Google Scholar]
- 14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2-ΔΔct method. Methods. 2001;25(6):402–408. [DOI] [PubMed] [Google Scholar]
- 15. Miranda KM, Espey MG, Wink DA. A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. [DOI] [PubMed] [Google Scholar]
- 16. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Bio Chem. 1976;72(5):24–254. [DOI] [PubMed] [Google Scholar]
- 17. Bancroft JD, Stevens A, Dawswon MP. Theory and Practice of Histological Techniques. 4th ed Churchill Livingstone; 1996:273–292. [Google Scholar]
- 18. Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7(3):4509–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Otegbeye F, Ojo E, Moreton S, et al. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS One. 2018;13(7):e0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haque S, Morris JC. Transforming growth factor-β: a therapeutic target for cancer. Hum Vaccin Immunother.2017;13(4):1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suzuki Y, Oretunua MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxplasma gondii. Science. 1985;240(4851):516–518. [DOI] [PubMed] [Google Scholar]
- 22. Sher A, Yap G, Aliberti J. Induction and regulation of IL-12-dependent host resistance to toxoplasma gondii. Immunol Res. 2003;27(3):521–522. [DOI] [PubMed] [Google Scholar]
- 23. Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasmagondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62(6):2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen JL, Ge YY, Zhang J, et al. The dysfunction of CD4(+)CD25(+) regulatory T cells contributes to the abortion of mice caused by Toxoplasma gondii excreted-secreted antigens in early pregnancy. PLoS One. 2013;8(4):e69012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulling PM, Olson KC, Hamele CE, et al. Dysregulation of the IFN-γ-STAT1 signaling pathway in a cell line model of large granular lymphocyte leukemia. PLoS One. 2018;13(2):e0193429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang W, Dong Z, Chen Y, et al. Small-molecule inhibitors targeting the DNA-binding domain of STAT3 suppress tumor growth, metastasis and STAT3 target gene expression in vivo. Onogene. 2016;35(3):783–792. [DOI] [PubMed] [Google Scholar]
- 27. Suganuma M, Okabe S, Marino MW, et al. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999;59(1):4516–4518. [PubMed] [Google Scholar]
- 28. Chai EZP, Siveen KS, Shanmugam MK, et al. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J. 2015;468(7):1–15. [DOI] [PubMed] [Google Scholar]
- 29. Medhat AM, Azab KHSH, Said MM, El Fatih NM, El Bakary NM. Antitumor and radio sensitizing synergistic effects of apigenin and cryptotanshinone against solid Ehrlich carcinoma in female mice. Tumor Biol. 2017;39(10):1010428317728480. [DOI] [PubMed] [Google Scholar]
- 30. Song K, Zhu F, Zhang HZ, et al. Tumor necrosis factor-α enhanced fusions between oral squamous cell carcinoma cells and endothelial cells via VCAM-1/VLA-4 pathway. Exp Cell Res. 2012;318(4):1707–1715. [DOI] [PubMed] [Google Scholar]
- 31. Wang FQ, Chen G, Zhu JY, et al. M2-polarised macrophages in infantile haemangiomas: correlation with promoted angiogenesis. J Clin Pathol. 2013;66(1):1058–1064. [DOI] [PubMed] [Google Scholar]
- 32. Zhao X, Sun X, Li XL. Expression and clinical significance of STAT3, P-STAT3, and VEGF-C in small cell lung cancer. Asian Pac J Cancer Prev. 2012;13(6):2873–2877. [DOI] [PubMed] [Google Scholar]
- 33. Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19(4):1296–1298. [DOI] [PubMed] [Google Scholar]
- 34. Noël A, Gutiérrez-Fernández A, Sounni NE, et al. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front Pharmacol. 2012;3(5):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao HH, Chu JH, Kwan HY, et al. Inhibition of the STAT3 signaling pathway contributes to apigenin mediated anti-metastatic effect in melanoma. Sci Rep. 2016;6(8):21731–21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hagemann T, Robinson SC, Schulz M, et al. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up- regulation of matrix metalloproteases. Carcinogenesis. 2004;25(7):1543–1549. [DOI] [PubMed] [Google Scholar]
- 37. Dilshara MG, Jayasooriya RG, Kang CH, et al. Methanol extract of Codium fragile inhibits tumor necrosis factor induced matrix metalloproteinase-9 and invasiveness of MDA-MB-231 cells by suppressing nuclear factor-kB activation. Asian Pac J Trop Med. 2016;9(5): 535–541. [DOI] [PubMed] [Google Scholar]
- 38. Liu JG, Zhao HJ, Yan-Juan L, Wang XL. Effect of selenium-enriched malt on VEGF and several relevant angiogenic cytokines in diethylnitrosamine-induced hepato carcinoma rats. J Trace Elem Med Biol. 2010;24(8):52–57. [DOI] [PubMed] [Google Scholar]
- 39. Pratheeshkumar P, Kuttan G. Nomilin inhibits tumor-specific angiogenesis by downregulating VEGF, NO and proinflammatory cytokine profile and also by inhibiting the activation of MMP-2 and MMP-9. Eur J Pharmacol. 2011;668(6):450–458. [DOI] [PubMed] [Google Scholar]
- 40. Khan MW, Saadalla A, Ewida AH, et al. The STAT3 inhibitor pyrimethamine displays anti-cancer and immune stimulatory effects in murine models of breast cancer. Cancer Immunol Immunother. 2018;67(2):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leng J, Butcher BA, Denkers EY. Dysregulation of macrophage signal transduction by toxoplasma gondii: past progress and recent advances. Parasite Immunol. 2009;31(8):717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zimmermann S, Murray PJ, Heeg K, Dalpke AH. Induction of suppressor of cytokine signaling-1 by toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-{gamma} signaling. J Immunol. 2006;176(2):1840–1847. [DOI] [PubMed] [Google Scholar]
- 43. Butcher BA, Kim L, Panopoulos A, et al. Cutting edge: IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-α in host macrophages. J Immunol. 2005;174(6):3148–3152. [DOI] [PubMed] [Google Scholar]
- 44. Hunter CA, Yu D, Gee M, et al. Cutting edge: systemic inhibition of angiogenesis underlies resistance to tumors during acute toxoplasmosis. J Immunol. 2001;166(5):5878–5881. [DOI] [PubMed] [Google Scholar]
- 45. Ellis LM, Fidler IJ. Angiogenesis and metastasis. Eur J Cancer. 1996;32A(5):2451–2460. [DOI] [PubMed] [Google Scholar]
- 46. Khan IA, Mac Lean JA, Lee FS, et al. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;(2):483–494. [DOI] [PubMed] [Google Scholar]
- 47. Strieter RM, Kunkel SL, Arenberg DA, et al. Interferon g-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210(8):51–57. [DOI] [PubMed] [Google Scholar]
- 48. Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of bax results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17(14):3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heimer S, Knoll G, Schulze-Osthoff K, Ehrenschwender M. Raptinal bypasses BAX, BAK, and BOK formitochondrial outer membrane permeabilization and intrinsic apoptosis. Cell Death Dis. 2019;10(4):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saxena M, Delgado Y, Sharma RK, et al. Inducing cell death in vitro in cancer cells by targeted delivery of cytochrome c via a transferrin conjugate. PLoS One. 2018;13(6):e0195542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi EJ, Kim GH. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 cells. J Clin Biochem Nutr. 2009;44(2):260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou Z, Zhu C, Cai Z, et al. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol Lett. 2018;15(1):7319–7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vasilev S, Ilic N, Gruden-Movsesijan A, et al. Necrosis and apoptosis in Trichinellaspiralis-mediated tumour reduction. Cent Eur J Immunol. 2015;40(2):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang G, Gao M. Influence of Toxoplasma gondii on in vitro proliferation and apoptosis of hepatoma carcinoma H7402Cell. Asian Pacific J Trop Med. 2016;9(3):63–66. [DOI] [PubMed] [Google Scholar]