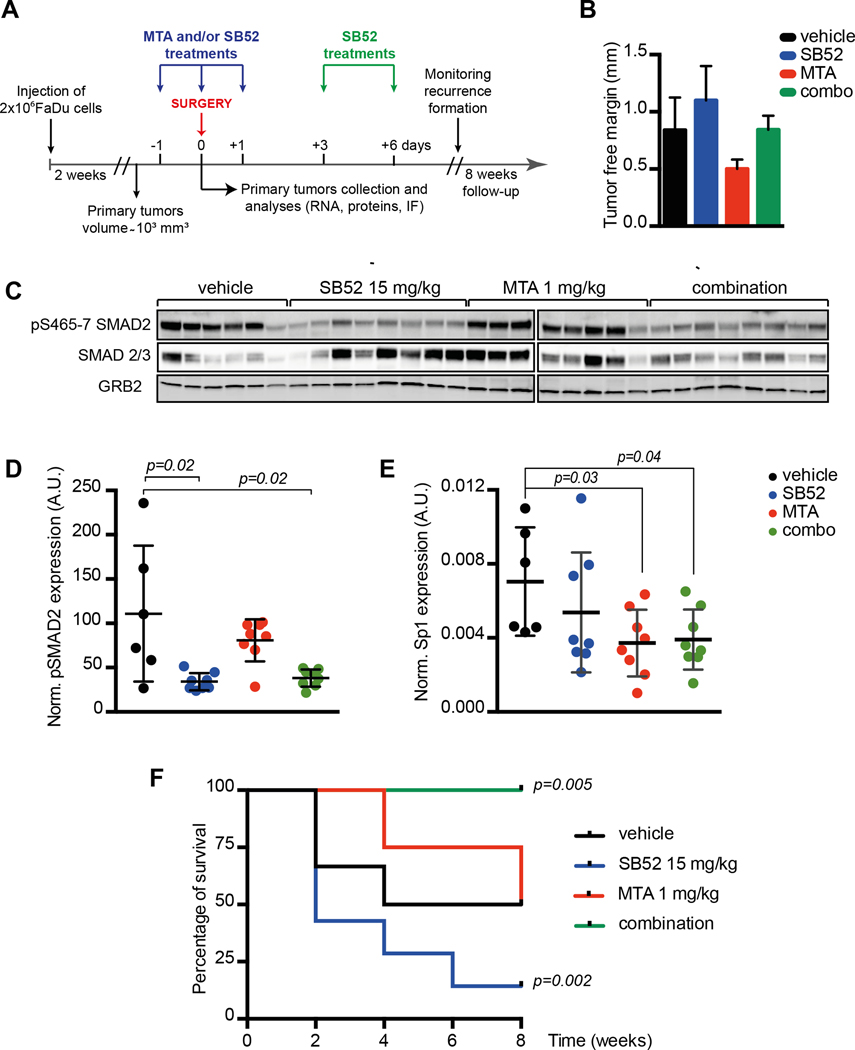
FIGURE 5. In vivo inhibition of SP1 and TGFβ pathway effectively reduces recurrence formation in mice.
A. Schematic representation of the experimental workflow used for the evaluation of recurrence formation in mice xenografted with HNSCC cells. Tumor bearing mice received the treatments the day before surgery (day −1), the day of surgery (day 0) and the days +1, +3 and +6 after surgery, as indicated. Mice have been then followed up to 8 weeks to monitor recurrence formation. Control mice were treated with the vehicle alone.
B The graph reports the evaluation of surgical resection margins in explanted tumors, measured with an optical microscope equipped with an ocular micrometer. Tumor free margins varied from 0.3 to 1.6 mm. No significant differences were observed among the four groups of treatment.
C Western blot analysis of tumors explanted from mice treated as described in (A) showing the expression and inhibition of pSMAD2 (S465/7) and SMAD2/3. GRB2 was used as loading control.
D/E Dot plot reporting the normalized expression of pSMAD2 (S465/7) (D) or SP1 mRNA (E) in tumors explanted at day 0 from mice treated as described in (A). Each dots in the graph represents a primary tumor that received the one dose of vehicle (black) MTA 1mg/kg (red), SB52 15mg/kg (blue) or their combination of MTA + SB52 (green).
F Disease-free survival of mice subjected to surgery to remove primary tumors (1000–1200 mm3) and then treated with vehicle (black line), MTA at 1mg/kg (red line), SB52 15mg/kg (blue line) or combination of MTA + SB52 (green line). Kaplan–Meier test has been used to calculate the significance of combo-treatment respect to control and to SB52-treated mice.