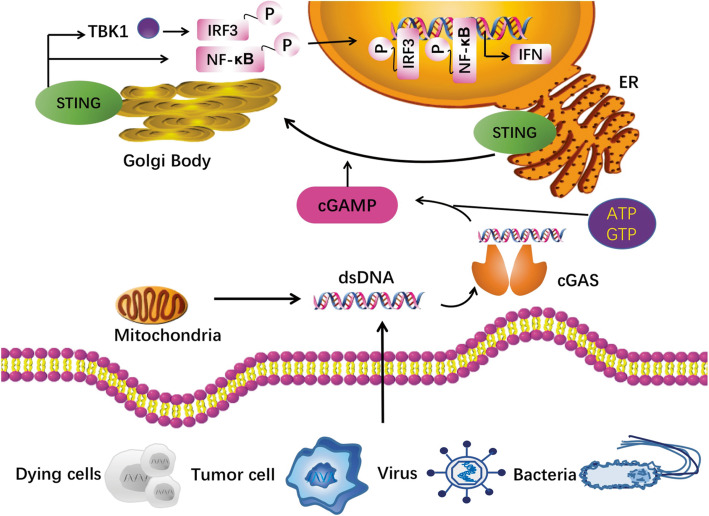
Fig. 2.
cGAS-STING pathway. Exogenous DNA from dying cell, tumor cell, virus and bacteria, and endogenous DNA leakage from mitochondria, interact with the cytosolic DNA sensor cGAS in a sequence-independent way, promoting a conformational change of cGAS to catalyze the formation of 2′,3′-cyclic GMP-AMP (cGAMP). The cGAS activation as well as cGAMP synthase activate protein STING, in which the STING undergoes endoplasmic reticulum (ER)-to-Golgi trafficking and tetramer formation via a higher-order oligomerization. Palmitoylation of STING in Golgi is proposed for TANK binding kinase 1 (TBK1) as well as interferon regulatory factor 3 (IRF3) recruitment. The STING tetramerization induces recruitment and activation of TBK1 dimers, and TBK1 transphosphorylates STING at its C-terminal domains for IRF3 activation. The IRF3 then displaces to the nucleus and induces immune-stimulated genes and type I IFN expression. The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling can also be activated by STING. Abbreviations: cGAMP, 2′,3′-cyclic GMP-AMP; ER, endoplasmic reticulum; IRF3, interferon regulatory factor 3; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TBK1, TANK-binding kinase 1