Abstract
Objective: The current authors observed enhanced cerebral blood flow (CBF) in the prefrontal cortex (PFC) in response to 100-Hz electroacupuncture (EA) stimulation of the ophthalmic branch of the trigeminal nerve. However, it is not yet clear if responsiveness to 100-Hz EA depends on stimulus intensity. This study examined the effects of stimulus strength on PFC CBF during 100-Hz EA of the ophthalmic branch of the trigeminal nerve.
Materials and Methods: Twelve subjects underwent 3 acupuncture sessions: I, control, no stimulation; II, 0.1 mA EA; and III, 0.2 mA EA). Needles were inserted 1 cm lateral of the head median line; the anterior insertion point was on the front hairline and the posterior insertion point was ∼7 cm behind the hairline. Stimulation frequency was set to 100-Hz. PFC CBF was measured in terms of oxygenated, deoxygenated, and total hemoglobin (OxyHb, DeoxyHb, TotalHb, respectively), using 16-channel (Ch) near-infrared spectroscopy.
Results: Stimulation of 0.2 mA was associated with significant elevation of OxyHb levels in the 0.1 mA condition in Chs 6, 10, and 12. Ch 2–6, 10, 12 signals were notably higher than in the control condition. Stimulation of 0.2 mA and 0.1 mA were associated with significant declines in DeoxyHb levels, compared to the control condition in Ch 4. Finally, 0.2 mA stimulation in Chs 12 and 13 was associated with significant elevation of TotalHb levels in the control condition.
Conclusions: Using 0.2-mA stimulation, 100-Hz EA of the ophthalmic nerve enhances PFC CBF more strongly than 0.1-mA stimulation.
Keywords: 100-HzEA, 0.2 mA, block design, hemoglobin, frontal pole cortex, near-infrared spectroscopy
Introduction
In a previous study, the current authors and others found that 100-Hz electroacupuncture (EA) to the ophthalmic branch of the trigeminal nerve (i.e., the ophthalmic nerve: CN V1) enhanced cerebral blood flow (CBF) in the prefrontal cortex (PFC), a region that governs decision-making, working memory, and other higher-order functions.1 However, no researchers have yet considered how stimulus intensity regulates how well subjects respond to EA. Somatic afferent fibers of different groups have different current thresholds for EA-mediated activation (fiber group II < III < IV).2 Compared to the prestimulation state, CBF is increased after stimulation with an EA intensity exceeding 1.5 mA, which activates group III and IV fibers.3,4 Similar effects have even been observed in a related transcranial magnetic stimulation study, in which CBF in the left and right PFC was enhanced in a stimulus strength–dependent manner.5 According to these observations, the current authors predicted that responsiveness of CBF in the PFC to 100-Hz EA of the CN V1 would be dependent on stimulus intensity. Therefore, the objective of this study was to examine the effects of stimulus strength on PFC CBF during stimulation of the CN V1 with 100-Hz EA.
Materials and Methods
Subjects
Twelve healthy adults were enrolled in the experiment (age: 20.50 ± 0.67 years old, body mass index [BMI]: 23.10 ± 1.59 kg/m2). This study was approved by the ethics committee of Teikyo Heisei University (approval #30-099) and registered in the University Hospital Medical Information Network Clinical Trials Registry (reg. #UMIN000036312). Informed consent was obtained from all subjects in accordance with the provisions of the Declaration of Helsinki. All subjects signed informed written consent forms to participate. The recruitment period was from March 27, 2019 to June 28, 2019.
Experimental Design
The 12 subjects were scheduled for 3 acupuncture sessions (I, control; II, 0.1 mA; and III, 0.2 mA), with a 2-week washout period between sessions. Subjects were instructed to refrain from drinking alcohol from the day before a session, to refrain from caffeine consumption (e.g., coffee, black tea) on the same day as a session, and to eat nothing for 2 hours before the session. In addition, they were asked to arrive at the test site 20 minutes before the start of each session to acclimatize to the environment (e.g., temperature). Experiments were always carried out between 10:00 am and 1:00 pm at an ambient temperature of 24.5°C–25.5°C to avoid errors in blood flow and heart rate (HR) measurements. During recordings, all external factors that could potentially influence the measurements (e.g., sounds) were minimized. Sessions had a block design, as follows: the subject was seated and rested for 5 minutes; then received 5 cycles of EA (1 minute each) + rest (1 minute); 15-minute total. In the control session, recordings were made during the 5-minute resting period while each subject sat and during the five 1-minute no-stimulus blocks (15 minutes).
EA
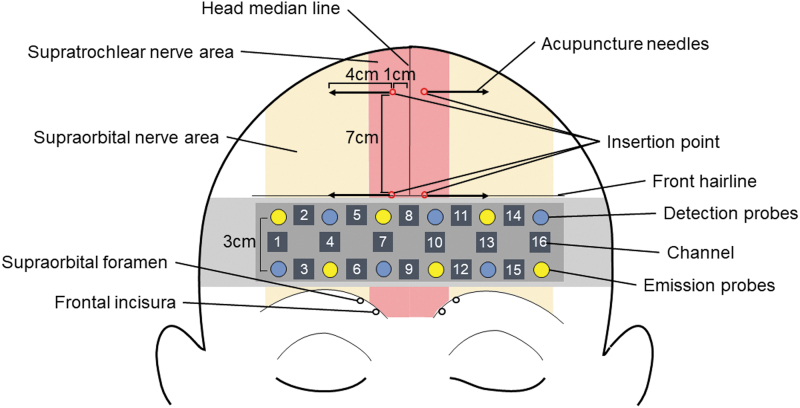
The stainless-steel acupuncture needles were 50 mm long and had a diameter of 0.18 mm (SEIRIN Corp. Shizuoka, Japan). The 2 insertion points were set at 1 cm lateral to the head median line; the anterior insertion point was on the front hairline and the posterior insertion point was 7 cm behind the front hairline (Fig. 1). These insertion points correspond to the supraorbital and supratrochlear nerves of the CN V1. The stainless-steel acupuncture needles were inserted horizontally into the insertion points on both sides at an approximate depth of 40 mm under the skin. Electrical stimulation (frequency: 100 Hz; pulse width: 0.25 milliseconds) was applied using an EA stimulator (Ohm Pulser LFP-2000e; Zen Iryoki Corp., Tokyo, Japan). EA stimulation was administered by a licensed acupuncturist with more than 4 years of experience.
FIG. 1.
The 4 insertion points of the acupuncture needles and the sites of the 16-channel (Ch) near-infrared spectroscopy probes (Chs 1–16. The insertion points corresponded to the supraorbital and supratrochlear nerves of the CN V1, and the emission and detection probes were placed on the frontal pole region according to the international 10–20 system. Color images are available online.
Near-Infrared Spectrometry
CBF was measured in terms of oxygenated hemoglobin (OxyHb), deoxygenated hemoglobin (DeoxyHb), and total hemoglobin (TotalHb) levels, using 16-channel (Ch) near-infrared spectroscopy (OEG-16; Spectratech Corp., Tokyo, Japan) as shown in Figure 1. OxyHb and DeoxyHb levels were calculated based on the absorptions of 840-nm and 770-nm light, respectively. Emission and detection probes were placed 3.0 cm apart, allowing the device to monitor the surface of the cerebral cortex over a span of 2–2.5 cm directly below the scalp. Chs corresponded to the following brain regions: Chs 1, 2, 14, and 16: frontal-pole cortex (FPC) and dorsolateral PFC (DL-PFC); Chs 3 and 15: FPC and inferior frontal gyrus (IFG); and Chs 4, 5, 6, 7, 10, 11, 12, and 13: FPC. The averaged signals of the 10 seconds before stimulation and the 40–50 seconds after stimulation were respectively defined as the pre- and poststimulation baselines. The sampling interval was 0.65 seconds. Hemoglobin levels in the right and left PFC were evaluated using the signals from Chs 1–7 and 10–16, respectively. Chs 8 and 9 were not evaluated as they were present on the head median line.
Heart Rate
Heart rate (HR) was measured using a Marquette Holter recorder (LRR-03; GMS Corp., Tokyo, Japan) via a disposable electrocardiograph monitoring electrode (Vitrode Bs 150; Nippon Koden Corp., Tokyo, Japan). During the 0.1 mA and 0.2 mA sessions, HR was recorded during the 5-minute rest period, and during 5 × 1-minute EA blocks. During the control session, HR was recorded during the 5-minute rest period and during the 5 × 1-min no-stimulus blocks. HR was calculated as the mean of these 5 1-minute values; changes were evaluated, compared to the rest period.
Statistical Analysis
All data are presented as the mean ± standard deviation. OxyHb, DeoxyHb, TotalHb, and HR changes were analyzed using the Kruskal–Wallis test followed by the Mann–Whitney-U test. Statistical significance was set at P < 0.05 for all tests.
Results
Of the initially recruited 12 subjects, 4 subjects dropped out of the study; only 8 subjects completed the entire study.
Sensations During EA Stimulation
The sensations during EA stimulation of the ophthalmic nerve varied between 0.1 and 0.2 mA; 3 subjects did not feel 0.1-mA EA stimulation, 3 subjects felt it slightly, and 2 subjects felt it distinctly. However, all subjects felt the 0.2-mA EA stimulation: 1 subject felt it slightly and 7 subjects felt it distinctly.
OxyHb
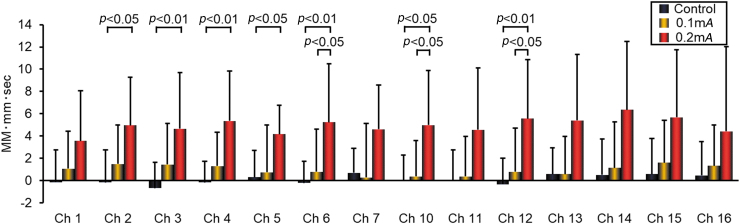
Figure 2 shows the OxyHb levels in Chs 1–7 and 10–16, which corresponded to the left and right PFC, respectively (R-PFC, L-PFC).
FIG. 2.
Oxygenated hemoglobin levels in the prefrontal cortex, measured using near-infrared spectroscopy, during a 1-minute session of electroacupuncture or a 1-minute rest, averaged over a 5-round stimulus cycle. Data from 8 healthy subjects. Data are presented as the mean ± standard deviation. MM-mm, millimole millimeter; sec, second; Ch, Channel;. Color images are available online.
In the R-PFC, 0.2-mA stimulation was associated with significant elevation of OxyHb levels, compared to the 0.1-mA condition in Ch 6 (P < 0.05). In addition, the Ch 2–6 signal was notably higher than that in the control condition (Chs 2 and 5: P < 0.05; Chs 3, 4, and 6: P < 0.01).
In the L-PFC, 0.2-mA stimulation was associated with significant elevation of OxyHb levels, compared to the 0.1-mA condition in Chs 10 and 12 (P < 0.05). Compared with the control condition, Chs 10 and 12 signals were significantly higher (P < 0.05 and P < 0.01, respectively).
DeoxyHb
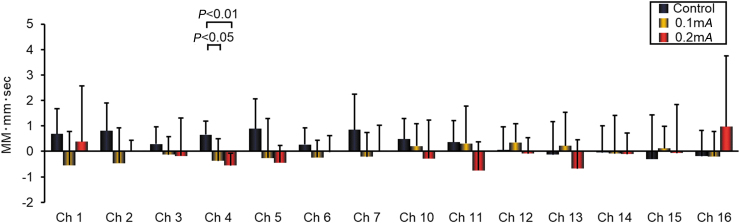
Figure 3 shows the DeoxyHb levels in Chs 1–7 and 10–16 that corresponded to the R-PFC and L-PFC, respectively. In the R-PFC, 0.2-mA and 0.1-mA stimulation was associated with significant decline in DeoxyHb levels, compared to the control condition in Ch 4 (P < 0.01 and P < 0.05, respectively). There were no significant changes in DeoxyHb levels in any channel in either EA condition in the L-PFC, compared to each other or to the control condition.
FIG. 3.
Deoxygenated hemoglobin levels in the prefrontal cortex, measured using near-infrared spectroscopy, during a 1-minute session of electroacupuncture or 1-minute rest, averaged over a 5-round stimulus cycle. Data from 8 healthy subjects. Data are presented as the mean ± standard deviation. MM-mm, millimole millimeter; sec, second; Ch, Channel. Color images are available online.
TotalHb
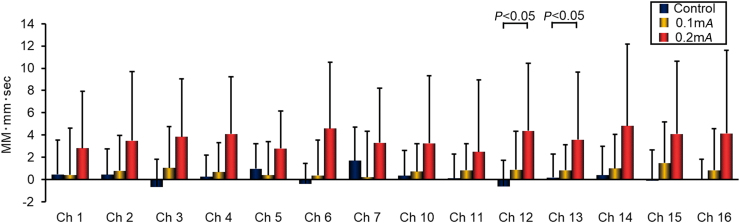
Figure 4 shows the data for TotalHb levels in Chs 1–7 and 10–16 that corresponded to the R-PFC and L-PFC.
FIG. 4.
Total hemoglobin levels in the prefrontal cortex, measured using near-infrared spectroscopy, during a 1 minute session of electroacupuncture or 1-minute rest, averaged over a 5-round stimulus cycle. Data from eight healthy subjects. Data are presented as the mean ± standard deviation. MM-mm, millimole millimeter; Ch, Channel. Color images are available online.
In the R-PFC, there were no significant changes in TotalHb levels in any channel in either EA condition, compared to each other or to the control condition. In the L-PFC, 0.2-mA stimulation in Chs 12 and 13 was associated with significant elevation of Total Hb levels, compared to the control condition (P < 0.05).
HR Changes
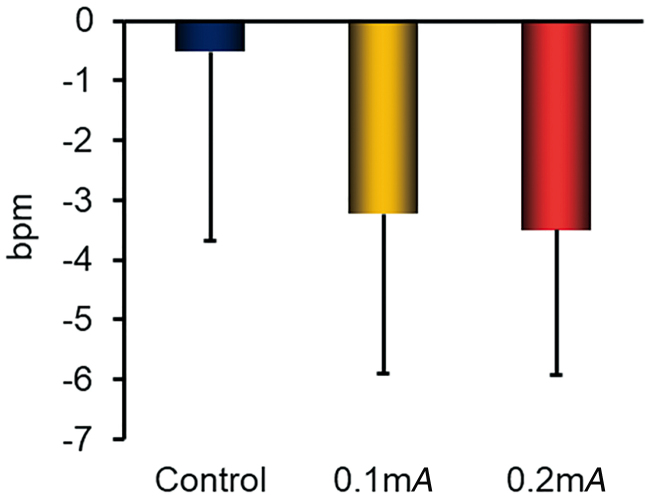
Figure 5 presents the HR changes. There were no significant changes in HR in either EA condition, compared with each other or with the control condition.
FIG. 5.
Heart rate changes, measured using a Marquette Holter recorder, during a 1-minute session of electroacupuncture or a 1-minute rest, averaged over a 5-round stimulus cycle. Changes were evaluated, compared to the 5-minute rest period. Data from 8 healthy subjects. Data are presented as the mean ± standard deviation. bpm, beats per minute. Color images are available online.
Adverse Events
No adverse events occurred during the insertions in this cohort.
Discussion
This study examined the effects of stimulus strength on PFC CBF during 100-Hz EA stimulation (100 Hz) of the ophthalmic nerve. The primary outcome of interest, namely, CBF, was analyzed in terms of levels of OxyHb, DeoxyHb, and TotalHb levels. Stronger EA stimulation (0.2 mA) produced higher levels of OxyHb in the FPC than slight EA stimulation (0.1 mA).
DeoxyHb tends not to follow CBF as closely as OxyHb or TotalHb. This is presumably why the EA-induced changes were less commonly observed in most channels.
Subjects felt the EA stimulation of the ophthalmic nerve more distinctly at 0.2 mA than at 0.1 mA. This intensity was also associated with greater CBF enhancement than 0.1 mA EA, which subjects felt faintly if at all. In a previous study, the current authors and others produced an increase in CBF by stimulating the trigeminal nerve; the intensity of the current was set so that no pain was felt but the stimulus was perceptible.1 Thus, it was possible to conclude that, to enhance the PFC CBF, EA must be applied at an intensity strong enough for the subject to experience the sensation distinctly.
Characteristically, the biologic reaction is enhanced with increases in the stimulus transmitted via somatic afferent nerve fibers. In previous studies using anesthetized rats, somatic afferent fibers of different groups had different current thresholds for EA-mediated activation (group II < III < IV).2 EA does not increase CBF with weak stimuli (≤ 0.5 mA) that activate group II fibers, but increases CBF with strong stimuli (≥ 1.5 mA) that activate groups III and IV fibers.3,4 A 0.2-mA EA stimulus enhanced the CBF of PFC more strongly than 0.1 mA stimulation in this study. Therefore, although different from the stimuli intensity used in the previous study, 0.1 mA might not have increased the CBF of PFC as it mainly activated group II fibers, and 0.2 mA might have increased the CBF of PFC as it mainly activated groups III and IV fibers. In addition, polymodal receptors are localized in the skin and muscles and respond to acupuncture stimuli.6
Aδ fibers, corresponding to group III fibers, control muscle polymodal receptors, and C fibers, corresponding to group IV fibers, control skin and muscle polymodal receptors.7,8 The excitability of these polymodal receptors is closely related to the response through the autonomic nervous system and increases depending on the intensity of the stimulus.9 For the above reasons, the 0.2-mA EA stimulus in this study might have increased the excitability of the polymodal receptors in group III (Aδ) fibers and group IV (C) fibers by >0.1 mA. In addition, the reflex arch of the trigeminal nerve and the postganglionic fibers of the facial nerve are involved in cerebral vasodilation.10 Therefore, it is likely that the excitability of the trigeminal polymodal receptors induced by a 0.2-mA EA stimulus increased CBF by dilating blood vessels distributed in the prefrontal cortex through the postganglionic fibers of the facial nerve.
Blood is primarily supplied to the PFC by the anterior and middle cerebral arteries, which branch from the internal carotid artery. These 3 vessels are mainly controlled by primary sensory neurons of the CN V1 and postganglionic fibers of the facial nerve (CN VII).11–13 Researchers have speculated that stimulating CN V1 can trigger vasodilation via an axon-reflex mechanism.14 This nerve also innervates the trigeminocervical complex, and synapses with the pterygopalatine ganglion (PPG) via the superior salivatory nucleus.10,14–16 These routes may activate parasympathetic nerve fibers originating in the pterygopalatine and otic ganglia, leading to vasodilation.13,17,18
CBF increases in response to brain vasodilation, which is regulated by extrinsic (peripheral) and intrinsic (central) nerves.19 Exogenous vasodilation in the brain is regulated by primary sensory neurons of the trigeminal nerve (CN V) and postganglionic fibers of CN VII.11,17 In the former case, it is induced by factors such as calcitonin gene–related peptide, substance P, and pituitary adenylate cyclase-activated peptide.11,20,21 CN VII postganglionic fibers originate from the pterygopalatine and otic ganglia; they induce vasodilation by releasing acetylcholine (ACh) and vasoactive intestinal peptide (VIP).17,18 Originating in the superior salivatory nucleus, the greater petrosal nerve triggers cerebral vasodilation by synapsing with nitric oxide (NO)–ergic neurons in the PPG, which causes them to release NO from their terminals.13,22,23 In addition, the dural arteries are innervated by postganglionic fibers from the facial nerve, which originates from trigeminal primary sensory neurons and the PPG; trigeminal stimulation may increase blood flow in the dural arteries via an axon-reflection mechanism.24,25 Sympathetic cerebral vasodilation has also been reported to be a compensatory response to vasoconstriction by adrenergic neurons originating in the superior cervical ganglion.26,27
Endogenous vasodilation in the brain is regulated by γ–aminobutyric acid (GABA)–ergic interneurons in the cerebral cortex, which are innervated by cholinergic neurons originating in the basal forebrain. GABA is the major neurotransmitter involved; however, ACh, NO, and VIP also contribute to this process.3,4,28
Therefore, there are 4 putative mechanisms—3 extrinsic and 1 intrinsic—explaining how 0.2-mA EA stimulation of the ophthalmic nerve might enhance CBF in the PFC via vasodilation of the internal carotid and anterior and middle cerebral arteries. These potential mechanisms are as follows:
-
(1)
ACh and VIP are released by postganglionic fibers of the facial nerve, which originate in the PPG.
-
(2)
NO is released by NO-ergic neurons, which synapse in the PPG.
-
(3.)
Calcitonin gene–related peptide, substance P, and pituitary adenylate cyclase–activated peptide are released by trigeminal primary sensory neurons via the axonal reflex.
-
(4)
Cholinergic nerves in the cerebral medulla release ACh, and GABA-ergic interneurons in the cerebral cortex release GABA, ACh, NO, and VIP to regulate cerebral blood vessels (this is the one intrinsic mechanism).
Therefore, a mechanism involving these nerves can also be responsible for this process.
Trigeminal stimulation may lower the HR when the input to the spinal trigeminal nucleus innervates the parasympathetic cardiac nerves originating from the nucleus ambiguus and the dorsal nucleus of the vagus nerve. This phenomenon is known as the trigeminal cardiac reflex.29,30 These findings suggest that EA stimulation might have evoked this reflex, as subjects' HRs were lowered in both, 0.1-mA and 0.2-mA conditions, compared to the control condition (although nonsignificantly).
EA stimulation at 0.2 mA increased levels OxyHb in PFC areas, including the FPC. These regions govern cognitive functions such as decision-making and working memory.31 This suggests that, by enhancing PFC activity, 0.2-mA EA may improve cognitive function. The current authors plan to examine the effects of 0.2-mA EA stimulation on cognitive function, using cognitive tasks that involve verbal fluency and mental arithmetic, among others.
Few human studies have considered responsiveness to EA stimulation based on current intensity. The current authors believe that their findings could be used to determine appropriate current strengths for EA interventions in research settings and in clinical practice. Further studies are warranted on EA stimulus intensity using other parameters.
One limitation of this study was that, while near-infrared spectroscopy was assumed to measure blood flow in the cerebral cortex, these readings are also influenced by blood flow in the overlying dermal layers. Blood is supplied to the forehead by the supraorbital and supratrochlear arteries, which run alongside the nerves that originate from the ophthalmic nerve. This implies that the vasodilation evoked by EA in this region could have merely been within the skin and caused by a local axonal reflex, and that the resulting increase in volume was simplistically combined with the true CBF. Further detailed investigations should be performed, using a device that can isolate and remove the dermal component of blood flow.
Conclusions
EA of the ophthalmic nerve to a level of 100 Hz, using 0.2-mA, stimulation enhances PFC CBF more strongly than 0.1 mA. These study results suggest that, to enhance PFC CBF, EA must be conducted at an intensity strong enough for the subject to experience a distinct sensation.
Acknowledgments
The current authors would like to thank Editage for its English-language editing.
Author Disclosure Statement
No financial conflicts of interest exist.
Funding Information
The researchers received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors to conduct this study.
References
- 1. Waki H, Suzuki T, Tanaka Y, et al. . Effects of electroacupuncture to the trigeminal nerve area on the autonomic nervous system and cerebral blood flow in the prefrontal cortex. Acupunct Med. 2017;35(5):339–344 [DOI] [PubMed] [Google Scholar]
- 2. Ohsawa H, Yamaguchi S, Ishimaru H, et al. . Neural mechanism of pupillary dilation elicited by electro-acupuncture stimulation in anesthetized rats. J Auton Nerv Syst. 1997;64(2–3):101–106 [DOI] [PubMed] [Google Scholar]
- 3. Uchida S, Kagitani F, Suzuki A, et al. . Effect of acupuncture-like stimulation on cortical cerebral blood flow in anesthetized rats. Jpn J Physiol. 2000;50(5):495–507 [DOI] [PubMed] [Google Scholar]
- 4. Uchida S, Kagitani F. Effect of acupuncture-like stimulation on cortical cerebral blood flow in aged rats. J Physiol Sci. 2015;65(1):67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nahas Z, Lomarev M, Roberts DR, et al. . Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry. 2001;50(9):712–720 [DOI] [PubMed] [Google Scholar]
- 6. Kawakita K, Gotoh K. Role of polymodal receptors in the acupuncture-mediated endogenous pain inhibitory systems. Prog Brain Res. 1996;113:507–523 [DOI] [PubMed] [Google Scholar]
- 7. Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32(6):1025–1043 [DOI] [PubMed] [Google Scholar]
- 8. Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273(1):179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mizumura K, Kumazawa T. Reflex respiratory response induced by chemical stimulation of muscle afferents. Brain Res. 1976;109(2):402–406 [DOI] [PubMed] [Google Scholar]
- 10. Ishii H, Sato T, Izumi H. Parasympathetic reflex vasodilation in the cerebral hemodynamics of rats. J Comp Physiol B. 2014;184(3):385–399 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki N, Hardebo JE, Kåhrström J, et al. . Effect on cortical blood flow of electrical stimulation of trigeminal cerebrovascular nerve fibres in the rat. Acta Physiol Scand. 1990;138(3):307–316 [DOI] [PubMed] [Google Scholar]
- 12. Nakai M, Tamaki K, Ogata J, et al. . Parasympathetic cerebrovasodilator center of the facial nerve. Circ Res. 1993;72(2):470–475 [DOI] [PubMed] [Google Scholar]
- 13. Toda N, Tanaka T, Ayajiki K, et al. . Cerebral vasodilatation induced by stimulation of the pterygopalatine ganglion and greater petrosal nerve in anesthetized monkeys. Neuroscience. 2000;96(2):393–398 [DOI] [PubMed] [Google Scholar]
- 14. Chiluwal A, Narayan RK, Chaung W, et al. . Neuroprotective effects of trigeminal nerve stimulation in severe traumatic brain injury. Sci Rep. 2017;7(1):6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. May A. Cluster headache: Pathogenesis, diagnosis, and management. Lancet. 2005;366(9488):843–855 [DOI] [PubMed] [Google Scholar]
- 16. Akerman S, Holland PR, Lasalandra MP, et al. . Oxygen inhibits neuronal activation in the trigeminocervical complex after stimulation of trigeminal autonomic reflex, but not during direct dural activation of trigeminal afferents. Headache. 2009;49(8):1131–1143 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki N, Hardebo JE, Kåhrström J, et al. . Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab. 1990;10(3):383–391 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki N, Hardebo JE, Owman C. Origins and pathways of choline acetyltransferase-positive parasympathetic nerve fibers to cerebral vessels in rat. J Cereb Blood Flow Metab. 1990;10(3):399–408 [DOI] [PubMed] [Google Scholar]
- 19. Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100(3):1059–1064 [DOI] [PubMed] [Google Scholar]
- 20. Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular nerves storing substance P and calcitonin gene–related peptide in rat. Neuroscience. 1989;31(2):427–438 [DOI] [PubMed] [Google Scholar]
- 21. Baeres FM, Møller M. Origin of PACAP-immunoreactive nerve fibers innervating the subarachnoidal blood vessels of the rat brain. J Cereb Blood Flow Metab. 2004;24(6):628–635 [DOI] [PubMed] [Google Scholar]
- 22. Morita-Tsuzuki Y, Hardebo JE, Bouskela E. Inhibition of nitric oxide synthase attenuates the cerebral blood flow response to stimulation of postganglionic parasympathetic nerves in the rat. J Cereb Blood Flow Metab. 1993;13(6):993–997 [DOI] [PubMed] [Google Scholar]
- 23. Toda N, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev. 2003;55(2):271–324 [DOI] [PubMed] [Google Scholar]
- 24. Bolay H, Reuter U, Dunn AK, et al. . Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 2002;8(2):136–142 [DOI] [PubMed] [Google Scholar]
- 25. Messlinger K, Hanesch U, Kurosawa M, et al. . Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can J Physiol Pharmacol. 1995;73(7):1020–1024 [DOI] [PubMed] [Google Scholar]
- 26. Nielsen KC, Owman C. Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res. 1967;6(4):773–776 [DOI] [PubMed] [Google Scholar]
- 27. Sercombe R, Lacombe P, Aubineau P, Mamo H, Pinard E. Eeynier-Rebuffel AM, Seylaz J. Is there an active mechanism limiting the influence of the sympathetic system on the cerebral vascular bed? Evidence for vasomotor escape from sympathetic stimulation in the rabbit. Brain Res. 1979;164:81–102 [DOI] [PubMed] [Google Scholar]
- 28. Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res. 2004;145:171–178 [DOI] [PubMed] [Google Scholar]
- 29. Lang S, Lanigan DT, van der Wal M. Trigeminocardiac reflexes: maxillary and mandibular variants of the oculocardiac reflex. Can J Anaesth. 1991;38(6):757–760 [DOI] [PubMed] [Google Scholar]
- 30. Bauer DF, Youkilis A, Schenck C, et al. . The falcine trigeminocardiac reflex: Case report and review of the literature. Surg Neurol. 2005;63(2):143–148 [DOI] [PubMed] [Google Scholar]
- 31. Tsujimoto S, Genovesio A, Wise SP. Evaluating self-generated decisions in frontal pole cortex of monkeys. Nat Neurosci. 2010;13(1):120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]